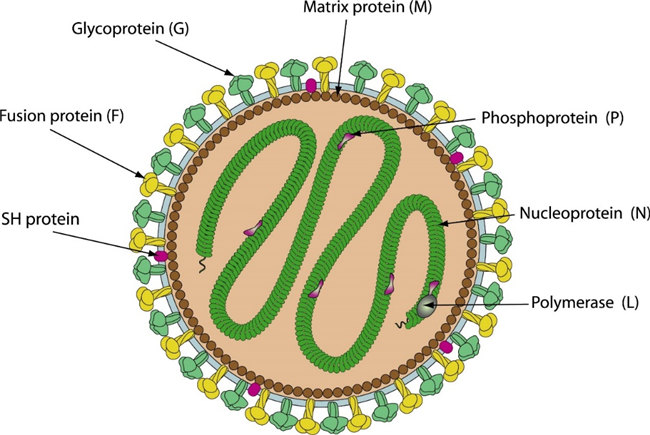
Fig 1. Schematic representation of human metapneumovirus (HMPV) (viralzone)
Human metapneumovirus (HMPV) is a leading cause of acute respiratory infections, particularly in children, immunocompromised patients, and the elderly. Although the virus was discovered in 2001, however, some studies show that HMPV has been circulating undetected for many decades. It is thought to spread through direct or close contact with infected individuals or contaminated objects (fomites). Despite significant advances since its discovery 23 years ago, there are currently no U.S. Food and Drug Administration (FDA)-approved antivirals or vaccines for HMPV. However, research into vaccine development is ongoing, and subunit vaccines that contain partial or full-length viral proteins, particularly the HMPV F protein, have shown promising results. Several studies of recombinant F protein report protective immunity without enhanced disease in cotton rats, hamsters, and non-human primates. Another non-replicating vaccine approach involves the use of virus-like particles that utilize HMPV M and F proteins expressed in human embryonic kidney epithelial cells or generated using retroviral vectors.
Human metapneumovirus (HMPV) is an enveloped, non-segmented, negative-sense, single-stranded RNA virus. The genome is 13.3 kilobase pairs in length and is composed of eight genes encoding nine proteins: nucleoprotein (N), phosphoprotein (P), matrix protein (M), fusion protein (F), matrix-2 proteins (M2-1 and M2-2), small hydrophobic (SH) protein, glycoprotein (G), and large (L) polymerase protein.
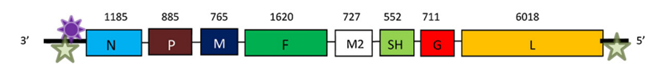
Fig 2. Genomic organization of hMPV (Feuillet et al., 2012)
Nucleoprotein (N)
The nucleoprotein in HMPV binds to the viral RNA, forming a protective complex and assembling into a left-handed helical nucleocapsid. Nucleocapsid assembly requires a pool of monomeric, RNA-free nucleoprotein, known as N0, which remains unassembled through interaction with the N-terminal portion of the polymerase cofactor P. This interaction prevents assembly until N0 is delivered to the sites of viral RNA synthesis. The nucleoprotein is crucial for protecting the RNA from nucleases and facilitating replication, thus maintaining the stability and integrity of the viral RNA genome.
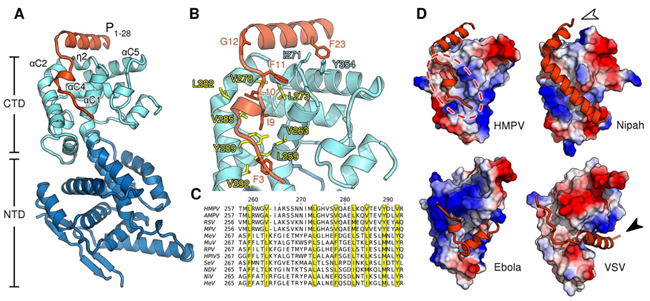
Fig 3. Structure of the HMPV N0-P complex (Renner et al., 2016)
matrix protein (M)
The matrix protein (M) is a crucial dimeric component that plays a key role in determining virion morphology by directing viral assembly and budding. It interacts with various viral and cellular components, such as nucleoprotein-RNA oligomers, lipid membranes, and the cytoplasmic tails of viral glycoproteins. M exhibits immunomodulatory properties by engaging with nucleic acids and host proteins, influencing nucleocytoplasmic trafficking, and inhibiting host cell transcription. Initially, M localizes in the nucleus to potentially inhibit transcription, but later, it traffics to the cytoplasm via host CRM1, where it associates with inclusion bodies, facilitating viral transcription and replication. Ultimately, M acts as a bridge between the nucleocapsid and the lipid bilayer during virus assembly and budding, with Ca²⁺ enhancing its stability.
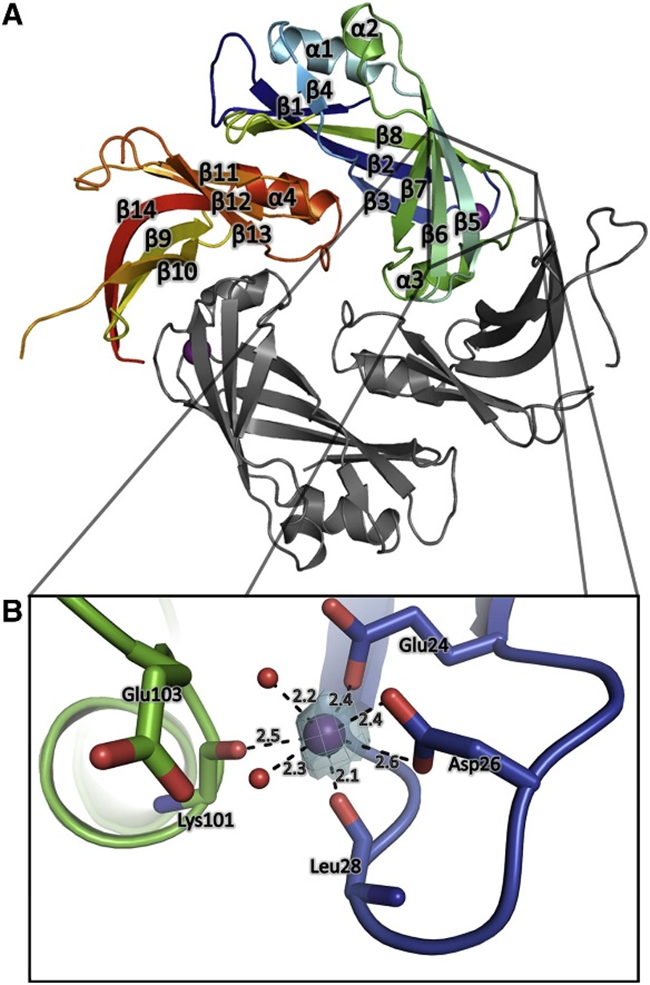
Fig 4. Crystal Structure of HMPV Matrix protein (Cedric et al., 2016)
Fusion protein (F)
The human metapneumovirus (hMPV) fusion protein (F) is a trimeric class I fusion protein essential for viral entry and a primary target for neutralizing antibodies and vaccine development. Initially synthesized as an inactive precursor (F0), it undergoes proteolytic cleavage by host cell proteases to form the fusion-competent prefusion conformation (Pre-F), which consists of disulfide-linked F1 and F2 subunits. The F protein is highly conserved among hMPV sublineages and facilitates viral attachment to host cells by binding to heparan sulfate. It exists in at least two conformational states—prefusion and post-fusion—and the neutralizing antibody response is primarily directed against the Pre-F form. During the fusion process, the F1 subunit adopts a trimer-of-hairpins structure, enabling membrane fusion. Activation can occur in cell culture with trypsin or naturally via serine proteases like TMPRSS2, underscoring the protein's crucial role in hMPV infection and its potential as a vaccine target.
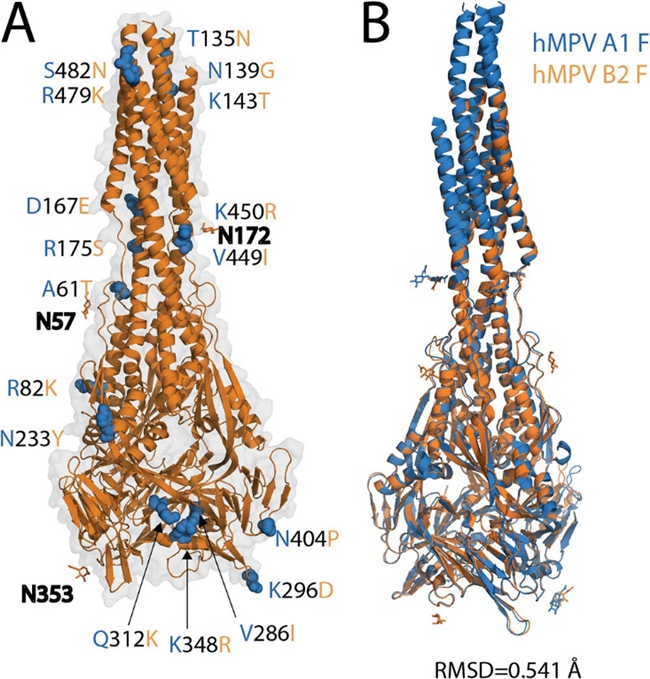
Fig 5. X-ray crystal structure of the postfusion hMPV B2 F protein (Huang et al., 2021)
Glycoprotein (G)
The glycoprotein G of human metapneumovirus (hMPV) is a crucial virulence factor involved in the pathogenesis of lung disease, including airway obstruction and airway hyperreactivity (AHR). It attaches the virion to the host cell membrane by interacting with glycosaminoglycans, initiating the infection. Unlike other paramyxovirus attachment proteins, G lacks both neuraminidase and hemagglutinating activities. In addition to facilitating attachment, G protein interacts with the host RIG-I receptor, inhibiting RIG-I-mediated signaling pathways to prevent the establishment of an antiviral state. Furthermore, G protein influences aspects of both innate and adaptive immunity, highlighting its role in the immune evasion strategies of hMPV.
Reference
1. Feuillet, F., Lina, B., Rosa-Calatrava, M., and Boivin, G. (2012). Ten years of human metapneumovirus research. J Clin Virol 53, 97-105.
2. Guo, L., Li, L., Liu, L., Zhang, T., and Sun, M. (2023). Neutralising antibodies against human metapneumovirus. The Lancet Microbe 4, e732-e744.
3. Huang, J., Chopra, P., Liu, L., Nagy, T., Murray, J., Tripp, R.A., Boons, G.-J., and Mousa, J.J. (2021). Structure, Immunogenicity, and Conformation-Dependent Receptor Binding of the Postfusion Human Metapneumovirus F Protein. Journal of Virology 95, 10.1128/jvi.00593-00521.
4. Jesse, S.T., Ludlow, M., and Osterhaus, A.D.M.E. (2022). Zoonotic Origins of Human Metapneumovirus: A Journey from Birds to Humans. Viruses 14, 677.
5. Leyrat, C., Renner, M., Harlos, K., Huiskonen, Juha T., and Grimes, Jonathan M. (2014). Structure and Self-Assembly of the Calcium Binding Matrix Protein of Human Metapneumovirus. Structure 22, 136-148.
6. Renner, M., Bertinelli, M., Leyrat, C., Paesen, G.C., Saraiva de Oliveira, L.F., Huiskonen, J.T., and Grimes, J.M. (2016). Nucleocapsid assembly in pneumoviruses is regulated by conformational switching of the N protein. eLife 5, e12627.
7. Shafagati, N., and Williams, J. (2018). Human metapneumovirus - what we know now. F1000Res 7, 135.
8. Whitehead, J.D., Decool, H., Leyrat, C., Carrique, L., Fix, J., Eléouët, J.-F., Galloux, M., and Renner, M. (2023). Structure of the N-RNA/P interface indicates mode of L/P recruitment to the nucleocapsid of human metapneumovirus. Nature Communications 14, 7627.
Host species: Human
Isotype: IgG1
Applications: ELISA, Neutralization
Accession: G3KCK8
Host species: Human
Isotype: IgG1, lambda2
Applications: ELISA, SPR
Expression system: Mammalian Cells
Host species: Rabbit
Isotype: IgG
Applications: ELISA, IHC, WB
Accession: C5MRS4
Host species: Human
Isotype: IgG1, lambda
Applications: ELISA, Neutralization
Host species: Human
Isotype: IgG1, lambda
Applications: ELISA, IF, SPR, WB
Accession: R4QK65, Q6WB98
Sample type: Plasma, Serum
Sample type: Plasma, Serum
Sample type: Plasma, Serum
Host species: Human
Isotype: IgG1, kappa
Applications: ELISA, Neutralization