Allergic diseases have become a significant public health issue in industrialized countries, affecting more than a quarter of the population. An allergic reaction is a pathological hypersensitivity of the immune system in response to specific antigens (such as repeated exposure to the same substance), leading to the production of IgE antibodies to defend against foreign invaders. This response is often accompanied by tissue inflammation, damage, and dysfunction. Common allergens include naturally occurring substances, such as dust mite excretion, pollen, fungal spores, and pet dander, as well as drugs and foods (such as nuts, milk, and seafood). Some individuals may experience severe allergic reactions, such as difficulty breathing or anaphylactic shock, which require prompt intervention and treatment.
The IgE receptor (FcεRI) on the surface of mast cells and basophils plays a crucial role in allergic reactions, with IgE binding to FcεRI regulating the differentiation and maturation of mast cells. However, the molecular mechanism of IgE binding to FcεRI remains unclear.
On October 23, 2024, a study by the team of Dr. Yigong Shi at Westlake University was published online in Nature. The research team utilized cryo-electron microscopy to successfully elucidate the structure of the IgE/FcεRI complex, revealing the molecular mechanism by which IgE regulates FcεRI and leads to allergic reactions.
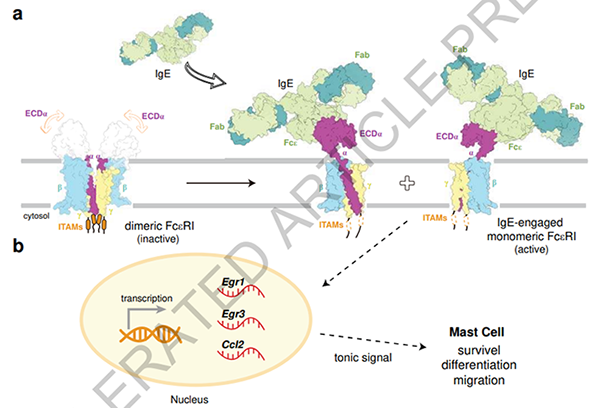
Figure 1. The Molecular Mechanism of IgE-Mediated FcεRI Activation Leading to Allergic Reactions
FcεRI consists of one α subunit, one β subunit, and two γ subunits, referred to as αβγ2. Research has found that FcεRI exists as a homo-dimer (αβγ2)2 on the surface of mast cells and basophils. Upon IgE binding, the dimeric FcεRI dissociates into two monomers, each binding to one IgE molecule, which immediately activates the transcription of Egr1 and Egr3, leading to an immune response. The dissociation of the FcεRI dimer is crucial for allergic reactions. Researchers fused a leucine zipper (GCN4) to the C-terminus of the α subunit to prevent the dissociation of the FcεRI dimer into monomers. They found that the GCN4-FcεRI dimer could only bind to one IgE molecule, and the activation of Egr1 and Egr3 transcription became slower.
AntibodySystem provides comprehensive allergy testing solutions, including recombinant allergen proteins and highly specific antibodies (including anti-IgE antibodies), to facilitate precise identification of common allergens such as dust mites, pet dander, pollen, and foods. The products include key allergens like dust mite Der f 1, cat allergen Fel d 1, and peanut Ara h 2, along with corresponding anti-IgE and various specific antibodies, supporting the detection and research of IgE-mediated allergic reactions, thereby aiding in the scientific management of allergy risks.
The list of star products is as follows:
Host species: Human
Isotype: IgG1
Applications: ELISA, FCM, Neutralization
Accession: P01563
Host species: Rabbit
Isotype: IgG
Applications: ELISA, IHC, WB
Accession: Q8H2B8
Applications: SDS-PAGE, WB, ELISA, Immunogen, Bioactivity testing in progress
Expression system: E. coli
Accession: Q8H2B8
Protein length: Ser21-Tyr138
Applications: SDS-PAGE, WB, ELISA, Immunogen, Bioactivity testing in progress
Expression system: E. coli
Accession: P25816
Protein length: Met1-Gln131
Applications: SDS-PAGE, WB, ELISA, Immunogen, Bioactivity testing in progress
Expression system: E. coli
Accession: P02662
Protein length: Arg16-Trp214
Applications: ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Expression system: E. coli
Accession: P00711
Protein length: Glu20-Leu142
Applications: ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Expression system: E. coli
Accession: P02662
Protein length: Arg16-Phe194
Host species: Alpaca (Lama pacos)
Isotype: VHH-8His-Cys-tag
Applications: ELISA
Accession: P00698
Host species: Mouse
Isotype: IgG2a
Applications: ELISA, WB
Accession: P00698
Host species: Mouse
Isotype: IgG2a, kappa
Applications: ELISA
Accession: P08176