Vesicular Stomatitis Virus (VSV)
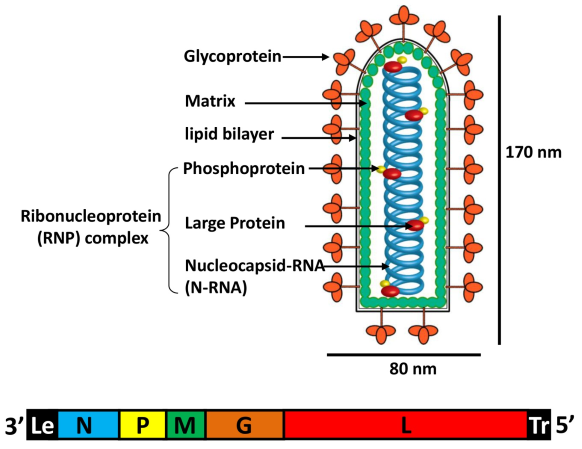
VSV virions are bullet-shaped particles 170 nm in length and 80 nm in diameter. The RNA genome of VSV consists of 11,161 nucleotides (nt) organized into five VSV genes encoding nucleocapsid (N), phospho- (P), matrix (M), glyco- (G), and large (L) proteins. Like all NNS RNA viruses, the genome is encapsidated with the N protein to form a nuclease-resistant helical N-RNA complex that is the functional template for mRNA synthesis as well as genomic RNA replication. The N-RNA complex is tightly associated with the viral RNA-dependent RNA polymerase (RdRp), which is comprised of the 241-kDa L protein catalytic subunit and the 29-kDa essential P protein cofactor, and results in the assembly of a viral ribonucleoprotein (RNP) complex. This structure contains the minimum virus encoded components of the VSV RNA synthesis machinery. The RNP complex is further surrounded by the M protein which plays a crucial role in virus assembly, budding, and maintenance of the structural integrity of the virus particle. The outer membrane of virion is the envelope composed of a cellular lipid bilayer. The transmembrane G protein is anchored in the viral envelope, which is essential for receptor binding and cell entry.
Matrix protein(P03519)
Function
Plays a major role in assembly and budding of virion, by recruiting cellular partners of the ESCRT complexes that play a key role in releasing the budding particle from the host membrane. Condensates the ribonucleocapsid core during virus assembly.
Inhibits mRNA nuclear export through direct interaction with host RAE1-NUP98 complex, thereby preventing interferon signaling and establishment of antiviral state in infected cells.
Induces cell-rounding, cytoskeleton disorganization and apoptosis in infected cell.
Inhibits host transcription, possibly through interaction with host DNA repair factor IIH/TFIIH GTF2H5 subunit.
RNA-directed RNA polymerase L(P03523)
Function
Responsible for RNA synthesis (replicase and transcriptase), cap addition, and cap methylation.
Performs also the polyadenylation of subgenomic mRNAs by a stuttering mechanism at a slipery stop site present at the end of viral genes (By similarity).
The template is composed of the viral RNA tightly encapsidated by the nucleoprotein (N) (Probable). The viral polymerase binds to the genomic RNA at the 3' leader promoter, thereby initiating either genome replication or mRNA transcription. In the transcription mode, the polymerase performs the sequential transcription of all mRNAs using a termination-reinitiation mechanism responding to gene start and gene end signals. Some polymerase disengage from the template at each gene junction, resulting in a decreasing abundance of transcripts from the 3' to the 5' end of the genome (By similarity).
The first gene is the most transcribed, and the last the least transcribed (Probable). The viral phosphoprotein helps the polymerase to engage the N-RNA template and acts as processivity factor.
Polyribonucleotidyl transferase (PRNTase) adds the cap structure when the nascent RNA chain length has reached few nucleotides.
Ribose 2'-O methylation of viral mRNA cap precedes and facilitates subsequent guanine-N-7 methylation, both activities being carried by the viral polymerase.
In the replication mode, the polymerase replicates the whole viral genome without recognizing the gene end transcriptional signals (By similarity).
The ability of the polymerase to override the gene end signals as it is producing the antigenome is probably due to replicative RNA becoming encapsidated with nucleoprotein as it is synthesized (By similarity).
Glycoprotein(P03522)
Function
Attaches the virus to host LDL receptors, inducing clathrin-dependent endocytosis of the virion.
In the endosome, the acidic pH induces conformational changes in the glycoprotein trimer, which trigger fusion between virus and endosomal membrane.
Phosphoprotein(P03520)
Function
Essential component of the RNA polymerase transcription and replication complex. Binds the viral ribonucleocapsid and positions the L polymerase on the template. May act as a chaperone for newly synthesized free N protein, so-called N0. Plays a role in virion assembly.
Nucleoprotein(P03521)
Function
Encapsidates the genome in a ratio of one N per nine ribonucleotides, protecting it from nucleases. The encapsidated genomic RNA is termed the NC and serves as template for transcription and replication. Nucleocapsid assembly is concommitant with replication, therefore viral replication depends on the intracellular concentration of free N, termed N0. All replicative products are resistant to nucleases.
Protein C'(P0C2X2)
Function
May play a role in viral pathogenesis or transmission by insects vectors.
Reference:
1.Whelan SPJ, Barr JN, Wertz GW. Transcription and replication of nonsegmented negative-strand RNA viruses. Biology of Negative Strand RNA Viruses: The Power of Reverse Genetics. 2004;283:61-119.
2.Abraham G, Banerjee AK. Sequential transcription of the genes of vesicular stomatitis virus. Proceedings of the National Academy of Sciences of the United States of America. 1976;73(5):1504-8.
3.Ball LA. Transcriptional mapping of vesicular stomatitis virus in vivo. Journal of virology. 1977;21(1):411-4.
4.Ball LA, White CN. Order of transcription of genes of vesicular stomatitis virus. Proceedings of the National Academy of Sciences of the United States of America. 1976;73(2):442-6.
5.Emerson SU, Wagner RR. Dissociation and reconstitution of the transcriptase and template activities of vesicular stomatitis B and T virions. Journal of virology. 1972;10(2):297-309.
6.Emerson SU, Yu YH. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. Journal of virology. 1975;15(6):1348-56.
7.Szilagyi JF, Uryvayev L. Isolation of an infectious ribonucleoprotein from vesicular stomatitis virus containing an active RNA transcriptase. Journal of virology. 1973;11(2):279-86.
8.Gaudin Y, Barge A, Ebel C, Ruigrok RW. Aggregation of VSV M protein is reversible and mediated by nucleation sites: implications for viral assembly. Virology. 1995;206(1):28-37.
9.Hammond C, Helenius A. Folding of VSV G protein: sequential interaction with BiP and calnexin. Science. 1994;266(5184):456-8.
Applications: SDS-PAGE, WB, ELISA, Immunogen, Bioactivity testing in progress
Expression system: E. coli
Accession: NP_041714.1
Protein length: Ile41-Lys229
Applications: ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Expression system: Mammalian Cells
Accession: P03522
Protein length: Lys17-Lys448
Applications: ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Expression system: Mammalian Cells
Accession: B5AGV3
Protein length: Lys17-Lys448
Host species: Rabbit
Isotype: IgG
Applications: ELISA, IHC, WB
Accession: B5AGV3
Applications: ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Expression system: Mammalian Cells
Accession: B5AGV3
Protein length: Met1-Ser467
Host species: Alpaca
Isotype: VHH-8His-Cys-tag
Applications: FCM, IP, SPR
Expression system: Mammalian Cells
Host species: Alpaca
Isotype: VHH-8His-Cys-tag
Applications: ELISA
Expression system: Mammalian Cells
Applications: ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Expression system: E. coli
Accession: B5AGV3
Protein length: Lys17-Ser467
Host species: Human
Isotype: IgG1, kappa
Applications: ELISA
Expression system: Mammalian Cells
Host species: Human
Isotype: IgG1, kappa
Applications: ELISA
Expression system: Mammalian Cells