(Niranjan, Arpana & Gupta, Pallavi. (2019). Recent Trends in Chikungunya Virus diagnosis. 5.)
Chikungunya virus (CHIKV) belongs to genus Alphavirus (family Togaviridae). It is transmitted by Aedes mosquitoes and has caused chikungunya fever, an emerging illness with systemic symptoms that include abrupt high fever, headache, skin rash, myalgia, and arthralgia.
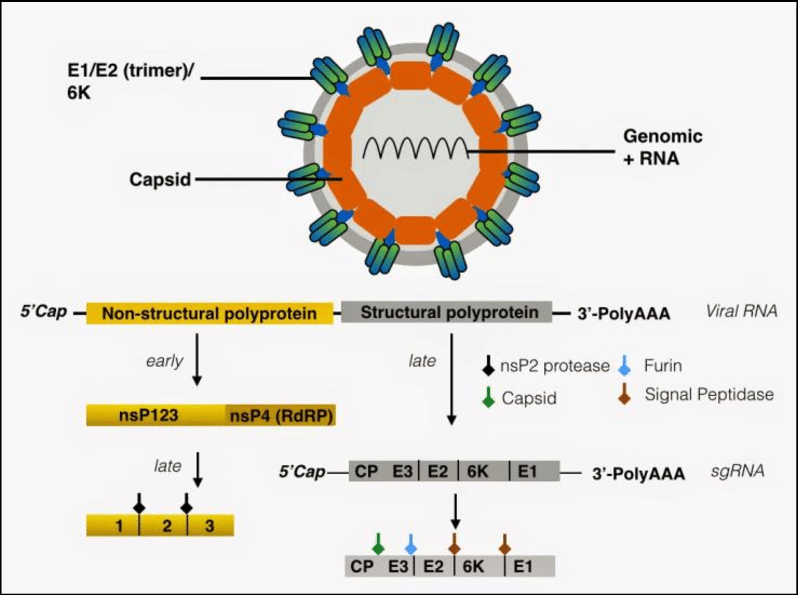
Chikungunya virus is a small (about 60–70 nm-diameter), spherical, enveloped, positive-strand RNA virus that is approximately 11 kb in length and codes for 9 proteins. The genome has two open reading frames (ORFs): the 5´ORF, translated from genomic RNA, encodes the nsP1, nsP2, nsP3, and nsP4 non-structural proteins, and the 3´ORF, genome is transcribed for subsequent translation into a polyprotein precursor containing the three structural proteins PE2 (the precursor of E3 and E2), E1, and the capsid protein.
Polyprotein P1234 (Q8JUX6)
Polyprotein P1234:
Inactive precursor of the viral replicase, which is activated by cleavages carried out by the viral protease nsP2.
Polyprotein P123:
The early replication complex formed by the polyprotein P123 and nsP4 synthesizes minus-strand RNAs (By similarity).
As soon P123 is cleaved into mature proteins, the plus-strand RNAs synthesis begins (By similarity).
mRNA-capping enzyme nsP1:
Cytoplasmic capping enzyme that catalyzes two virus-specific reactions: methyltransferase and guanylyltransferase (By similarity).
mRNA-capping is necessary since all viral RNAs are synthesized in the cytoplasm, and host capping enzymes are restricted to the nucleus (Probable). The enzymatic reaction involves a covalent link between 7-methyl-GMP and nsP1, whereas eukaryotic capping enzymes form a covalent complex only with GMP (By similarity).
nsP1 capping consists in the following reactions: GTP is first methylated into 7-methyl-GMP and then is covalently linked to nsP1 to form the m7GMp-nsP1 complex from which 7-methyl-GMP complex is transferred to the mRNA to create the cap structure (By similarity).
NsP1 is also needed for the initiation of the minus-strand RNAs synthesis (By similarity).
At the initiation of virus replication, mediates the assembly of the viral replication complex made of the non-structural proteins, the association of this complex with the inner face of the plasma membrane and the formation of membranous spherules that serve as replication complex factories (PubMed:33730549).
Forms the neck of these spherules with a central channel for mediating communication and the passage of RNA, nucleotides, and small proteins between the viral replication complex and the host cytoplasm (PubMed:33730549).
Palmitoylated nsP1 is remodeling host cell cytoskeleton, and induces filopodium-like structure formation at the surface of the host cell (PubMed:30404808).
Protease nsP2:
Multifunctional protein whose N-terminus is part of the RNA polymerase complex and displays NTPase, RNA triphosphatase and helicase activities (PubMed:21811589, PubMed:24407286).
NTPase and RNA triphosphatase are involved in viral RNA capping and helicase keeps a check on the dsRNA replication intermediates (By similarity).
The C-terminus harbors a protease that specifically cleaves the polyproteins and releases the mature proteins (PubMed:27845418, PubMed:26597768).
Required for the shutoff of minus-strand RNAs synthesis (By similarity).
Specifically inhibits the host IFN response by promoting the nuclear export of host STAT1 (PubMed:29925658).
Also inhibits host transcription by inducing the rapid proteasome-dependent degradation of POLR2A, a catalytic subunit of the RNAPII complex (PubMed:22514352).
The resulting inhibition of cellular protein synthesis serves to ensure maximal viral gene expression and to evade host immune response (Probable).
Non-structural protein 3:
Seems to be essential for minus-strand RNAs and subgenomic 26S mRNAs synthesis (By similarity).
Displays mono-ADP-ribosylhydrolase activity (PubMed:28143925, PubMed:28150709).
ADP-ribosylation is a post-translational modification that controls various processes of the host cell and the virus probably needs to revert it for optimal viral replication (PubMed:28143925, PubMed:28150709).
Binds proteins of G3BP family and sequesters them into the viral RNA replication complexes thereby inhibiting the formation of host stress granules on viral mRNAs (PubMed:25653451).
The nsp3-G3BP complexes bind viral RNAs and probably orchestrate the assembly of viral replication complexes, thanks to the ability of G3BP family members to self-assemble and bind DNA (PubMed:27509095, PubMed:27383630) (Probable).
RNA-directed RNA polymerase nsP4:
RNA dependent RNA polymerase (By similarity).
Replicates genomic and antigenomic RNA by recognizing replications specific signals. The early replication complex formed by the polyprotein P123 and nsP4 synthesizes minus-strand RNAs (By similarity).
The late replication complex composed of fully processed nsP1-nsP4 is responsible for the production of genomic and subgenomic plus-strand RNAs (By similarity).
Structural polyprotein (Q8JUX5)
Function
Capsid protein:
Forms an icosahedral capsid with a T=4 symmetry composed of 240 copies of the capsid protein surrounded by a lipid membrane through which penetrate 80 spikes composed of trimers of E1-E2 heterodimers (By similarity).
The capsid protein binds to the viral RNA genome at a site adjacent to a ribosome binding site for viral genome translation following genome release (By similarity).
Possesses a protease activity that results in its autocatalytic cleavage from the nascent structural protein (By similarity).
Following its self-cleavage, the capsid protein transiently associates with ribosomes, and within several minutes the protein binds to viral RNA and rapidly assembles into icosahedric core particles (By similarity).
The resulting nucleocapsid eventually associates with the cytoplasmic domain of the spike glycoprotein E2 at the cell membrane, leading to budding and formation of mature virions (By similarity).
In case of infection, new virions attach to target cells and after clathrin-mediated endocytosis their membrane fuses with the host endosomal membrane (By similarity).
This leads to the release of the nucleocapsid into the cytoplasm, followed by an uncoating event necessary for the genomic RNA to become accessible (By similarity).
The uncoating might be triggered by the interaction of capsid proteins with ribosomes (By similarity).
Binding of ribosomes would release the genomic RNA since the same region is genomic RNA-binding and ribosome-binding (By similarity).
Specifically inhibits interleukin-1 receptor-associated kinase 1/IRAK1-dependent signaling during viral entry, representing a means by which the alphaviruses may evade innate immune detection and activation prior to viral gene expression (By similarity).
Degrades host cyclic GMP-AMP synthase (CGAS) thereby inhibiting the cGAS-STING pathway (PubMed:33057424).
Assembly protein E3:
Provides the signal sequence for the translocation of the precursor of protein E3/E2 to the host endoplasmic reticulum. Furin-cleaved E3 remains associated with spike glycoprotein E1 and mediates pH protection of the latter during the transport via the secretory pathway. After virion release from the host cell, the assembly protein E3 is gradually released in the extracellular space.
Spike glycoprotein E2:
Plays a role in viral attachment to target host cell, by binding to the cell receptor MXRA8 (PubMed:29769725).
Synthesized as a p62 precursor which is processed by furin at the cell membrane just before virion budding, giving rise to E2-E1 heterodimer. The p62-E1 heterodimer is stable, whereas E2-E1 is unstable and dissociate at low pH. p62 is processed at the last step, presumably to avoid E1 fusion activation before its final export to cell surface. E2 C-terminus contains a transitory transmembrane that would be disrupted by palmitoylation, resulting in reorientation of the C-terminal tail from lumenal to cytoplasmic side. This step is critical since E2 C-terminus is involved in budding by interacting with capsid proteins. This release of E2 C-terminus in cytoplasm occurs lately in protein export, and precludes premature assembly of particles at the endoplasmic reticulum membrane.
6K protein:
Constitutive membrane protein involved in virus glycoprotein processing, cell permeabilization, and the budding of viral particles. Disrupts the calcium homeostasis of the cell, probably at the endoplasmic reticulum level. This leads to cytoplasmic calcium elevation. Because of its lipophilic properties, the 6K protein is postulated to influence the selection of lipids that interact with the transmembrane domains of the glycoproteins, which, in turn, affects the deformability of the bilayer required for the extreme curvature that occurs as budding proceeds. Present in low amount in virions, about 3% compared to viral glycoproteins.
Spike glycoprotein E1:
Class II viral fusion protein. Fusion activity is inactive as long as E1 is bound to E2 in mature virion. After virus attachment to target cell and endocytosis, acidification of the endosome would induce dissociation of E1/E2 heterodimer and concomitant trimerization of the E1 subunits. This E1 trimer is fusion active, and promotes release of viral nucleocapsid in cytoplasm after endosome and viral membrane fusion. Efficient fusion requires the presence of cholesterol and sphingolipid in the target membrane. Fusion is optimal at levels of about 1 molecule of cholesterol per 2 molecules of phospholipids, and is specific for sterols containing a 3-beta-hydroxyl group.
Reference:
1, Rausalu, K., Utt, A., Quirin, T. et al. Chikungunya virus infectivity, RNA replication and non-structural polyprotein processing depend on the nsP2 protease’s active site cysteine residue. Sci Rep 6, 37124 (2016).
2, Strauss, J. H. & Strauss, E. G. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 58, 491–562 (1994).
3, Rupp, J. C., Sokoloski, K. J., Gebhart, N. N. & Hardy, R. W. Alphavirus RNA synthesis and non-structural protein functions. J. Gen. Virol. 96, 2483–2500 (2015).
4, Salonen, A. et al. Properly Folded Nonstructural Polyprotein Directs the Semliki Forest Virus Replication Complex to the Endosomal Compartment. J. Virol. 77, 1691–1702 (2003).
5, Lemm, J. A., Rümenapf, T., Strauss, E. G., Strauss, J. H. & Rice, C. M. Polypeptide requirements for assembly of functional Sindbis virus replication complexes: a model for the temporal regulation of minus- and plus-strand RNA synthesis. EMBO J. 13, 2925–2934 (1994).
Isotype: Chikungunya virus (CHIKV) VLP (E1, E2 and Capsid)
Applications: Research Grade Biosimilar
Expression system: Mammalian cells
Accession: Q5XXP3
Host species: Rabbit
Isotype: IgG
Applications: ELISA, IHC, WB
Accession: Q8JUX5
Sample type: Plasma, Serum
Sample type: Plasma, Serum
Sample type: Plasma, Serum
Sample type: Plasma, Serum
Sample type: Plasma, Serum
Sample type: Plasma, Serum
Sample type: Plasma, Serum
Sample type: Plasma, Serum