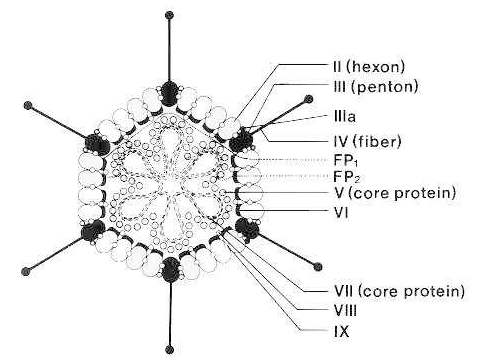
(From Brown DT, Westphal M, Burlingham BT et al.: Structure and composition of the adenovirus type 2 core. J. Virol. 16:366, 1975, with permission.)
Adenoviruses
The adenoviruses are common pathogens of humans and animals. Moreover, several strains have been the subject of intensive research and are used as tools in mammalian molecular biology. More than 100 serologically distinct types of adenovirus have been identified, including 49 types that infect humans. The family Adenoviridae is divided into two Genera, the mammalian adenoviruses (mastadenoviruses) and the avian adenoviruses (aviadenoviruses). The adenoviruses are named after the human adenoids, from which they were first isolated.
Several adenoviruses can cause respiratory and conjunctival diseases. In addition, a few types of human adenoviruses induce undifferentiated sarcomas in newborn hamsters and other rodents and can transform certain rodent and human cell cultures. There is currently no evidence that adenoviruses are oncogenic in humans, but the possibility remains of interest.
The adenovirus particle consists of an icosahedral protein shell surrounding a protein core that contains the linear, double-stranded DNA genome.(See figure 1) The shell, which is 70 to 100 nm in diameter, is made up of 252 structural capsomeres. The 12 vertices of the icosahedron are occupied by units called pentons, each of which has a slender projection called a fiber. The 240 capsomeres that make up the 20 faces and the edges of the isocahedron are called hexons because they form hexagonal arrays. The shell also contains some additional, minor polypeptide elements. The core particle is made up of two major proteins (polypeptide V and polypeptide VII) and a minor arginine-rich protein (μ). A 55 kDa protein is covalently attached to the 5′ ends of the DNA.
Protease(P03252 )
Function
Cleaves viral precursor proteins (pTP, pIIIa, pVI, pVII, pVIII, and pX) inside newly assembled particles giving rise to mature virions. Protease complexed to its cofactor slides along the viral DNA to specifically locate and cleave the viral precursors. Mature virions have a weakened organization compared to the unmature virions, thereby facilitating subsequent uncoating. Without maturation, the particle lacks infectivity and is unable to uncoat. Late in adenovirus infection, in the cytoplasm, may participate in the cytoskeleton destruction. Cleaves host cell cytoskeletal keratins K7 and K18.
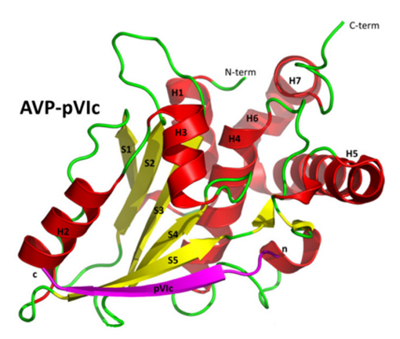
Crystal structure of AVP-pVIc complex. ((Blainey et al., 2013)
Adenovirus death protein (P24935 )
Function
Promotes the release of progeny virus from the host cell nucleus by accelerating the lysis and death of the host cell.
Function
Protein covalently bound to the viral DNA that acts as a primer for viral genomic replication by DNA strand displacement. Assembles on the viral origin of replication in an initiation complex with viral polymerase, DBP, host NFIA and host POU2F1/OCT1. During initiation, the polymerase covalently couples the first dCTP with Ser-580 of pTP. The terminal protein stimulates the template activity over 20 fold compared to protein-free templates. Neo-synthesized viral genomes are linked to two preterminal proteins, one for each 5' end. These new genomes are encapsidated in the nucleus, and during capsid maturation by viral protease, preterminal protein is first cleaved into intermediary (iTP), then into mature TP. May play a role in host nuclear matrix localization of genomic DNA.
Function
Component of the packaging machinery which encapsidates the viral DNA into preformed capsids and transcriptional activator of the viral major late promoter (MLP). Binds, along with packaging proteins 2 and 3, to the specific packaging sequence on the left end of viral genomic DNA and displays ATPase activity thereby providing the power stroke of the packaging machinery. The activity of packaging protein IVa2 is stimulated by protein 33K which acts as a terminase. May be the protein that pumps DNA into the capsid powered by ATP hydrolysis. Specifically binds to the 5'-CG-3' nucleotides of the repeats making up the packaging sequence. Component of the DEF-A and DEF-B transcription factors that bind downstream elements of the major late promoter (MLP), and stimulate transcription from the MLP after initiation of viral DNA replication. DEF-A is a heterodimer packaging proteins 1 and 2 and DEF-B is a homodimer of packaging protein 1.
Function
Binds and retains class I heavy chains in the endoplasmic reticulum during the early period of virus infection, thereby impairing their transport to the cell surface. Also delays the expression of class I alleles that it cannot affect by direct retention. Binds transporters associated with antigen processing (TAP) and acts as a tapasin inhibitor, preventing class I/TAP association. In consequence, infected cells are masked for immune recognition by cytotoxic T-lymphocytes (By similarity).
Function
During virus assembly, promotes hexon trimers nuclear import through nuclear pore complexes via an importin alpha/beta-dependent mechanism. By analogy to herpesviruses capsid assembly, might act as a chaperone to promote the formation of the icosahedral capsid.
Function
Plays a role in viral alternative pre-mRNA splicing. Activates dephosphorylation by protein phosphatase 2A of host SR proteins and converts their splicing properties. When expressed alone ex vivo, induces p53/TP53-independent apoptosis called cytoplasmic death. May mimic nutrient/growth signals to activate the host mTOR pathway.
Hexon protein
Hexon protein is encoded by the L3 gene of adenovirus. It is the most abundant protein in the capsid, with 720 subunits arranged as 240 trimers on a pseudo-T=25 icosahedral. The structure of the hexon trimer is characterized by three protruding towers formed by intertwined loops from all three hexon monomers. This extensive intertwining within the hexon trimer necessitates the assistance of an accessory protein, 100K (YVV40101), for proper folding of the hexon trimer. The hexon trimer has a large subunit interface, where each subunit clasps its neighboring subunit, resulting in a highly stable trimeric structure. The trimer is highly resistant to proteolysis and is so stable that it retains its physical and immunological characteristics even after exposure to 8 M urea.
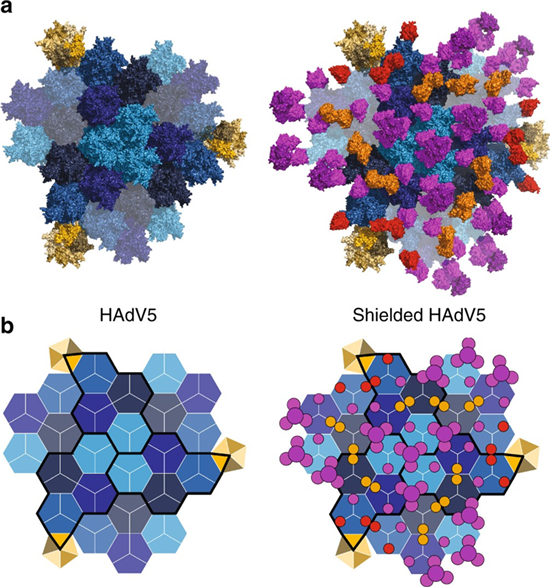
Structural and schematic representation of shield occupancy and stoichiometry on viral capsid (Schmid et al., 2018)
Penton protein
The penton protein is encoded by the L2 gene of adenovirus. Five penton base monomer subunits occupy each of the icosahedral vertices and are associated with the trimeric fiber protein. Each of the 20 facets of the capsid contains 12 hexon trimers and a penton at each vertex. The penton base protein serves as the secondary attachment site for cell surface integrins αvβ3 and αvβ5, triggering membrane permeabilization and virus internalization.
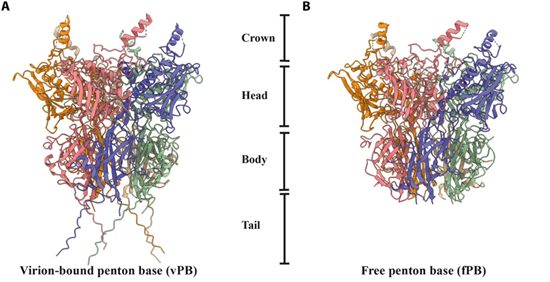
Cartoon representation of the Penton base (Rafie et al., 2021)
Fiber protein
The fiber protein, encoded by the L5 gene of adenovirus, forms trimeric fibers attached to the outer surface of the penton base pentamer, creating a non-covalent complex. Each fiber consists of three domains: the head, shaft, and N-terminal tail. The C-terminal globular head is responsible for fiber trimerization and attachment to the receptor at the host cell membrane. It can bind receptors in a non-stoichiometric manner, as shown by a cryo-EM study on the HAdV-B3 fiber head bound to desmoglein-2, where the trimeric head was found to bind either one or two copies of the receptor, but not three. The shaft domain features a variable number of repeats, providing structural flexibility and length to the fiber, while the N-terminal tail interacts with the penton base. The fiber knob, the distal part of the fiber, interacts with various host cell attachment receptors, including the coxsackie-adenovirus receptor (CAR), CD46 (membrane cofactor protein), sialic acid-containing oligosaccharides, GD1a glycan, and desmoglein-2 (DSG-2), facilitating virus attachment and entry into the host cell.
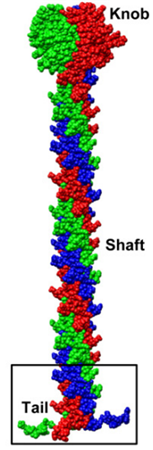
Model of the Trimeric Fiber (Liu et al., 2011)
100K
The 100K protein plays several critical roles essential for the successful completion of the late phases of the adenovirus life cycle. It is particularly important for the trimerization of the major capsid hexon protein, facilitating the generation of new virions within target host cells. As one of the key non-structural proteins (NSP) of the virus, 100K acts as a molecular chaperone, aiding in the folding of hexon and its assembly into capsomers. Studies have shown that hexon expressed alone in insect cells forms inclusion bodies, whereas co-expression with 100K results in the formation of soluble trimeric hexon that is indistinguishable from native hexon capsomers isolated from partially disrupted adenovirions. Additionally, the presence of 100K in complexes with hexon trimers further supports its role as a scaffold protein for hexon.
E1B-55K
The adenovirus E1B-55K protein is essential for productive viral infection and cellular transformation. It plays key roles by preventing cellular inhibition of viral genome replication, primarily through its interactions that counteract host defenses. E1B-55K also disrupts cellular processes by binding to the transactivation domain of the TP53 (p53) protein, thereby inactivating TP53's transcriptional activity. This action helps evade cellular responses such as cell cycle arrest and apoptosis. Moreover, E1B-55K is critical for efficient viral DNA replication, ensuring the replication of adenoviral genomes within infected cells. Additionally, E1B-55K contributes to the transformation of primary cells in culture, facilitating the establishment of a state conducive to viral persistence and propagation.
Host species: Mouse
Isotype: IgG1, kappa
Applications: ELISA, IHC, WB
Accession: P04133
Applications: ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Expression system: E. coli
Accession: P36851
Protein length: Confidential