Coxsackieviruses are a group of related enteroviruses within the Picornaviridae family, comprising non-enveloped, linear, positive-sense single-stranded RNA viruses. They belong to the genus Enterovirus, which includes some of the most common and significant human pathogens, primarily transmitted through the fecal-oral route.
Coxsackieviruses are categorized into group A and group B based on their pathogenic effects observed in neonatal mice. Group A viruses, including CVA16, are associated with flaccid paralysis due to generalized myositis, while group B viruses typically cause spastic paralysis from focal muscle injury and neuronal tissue degeneration. There are at least 23 recognized serotypes of group A (1-22, 24) and six serotypes of group B (1-6). Hand-foot-and-mouth disease (HFMD) is a serious public health threat to children in Asian-Pacific countries, resulting in millions of cases. Coxsackievirus A16 (CVA16) is one of the major pathogens associated with hand-foot-and-mouth disease.
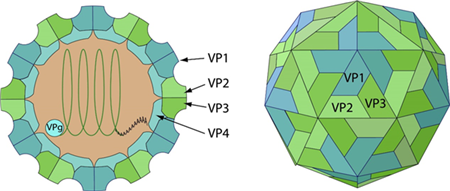
Fig 1. Schematic representation of Coxsackieviruses (viralzone)
The genome of CVA16 is a single stranded, positive sense RNA, containing approximately 7,410 nucleotides with a single open-reading-frame (ORF). The ORF has 6,579 nucleotides, encoding a polyprotein of 2,193 amino acids which is composed of 3 protein precursors: P1, P2, and P3. The P1 polyprotein precursor processed into 4 structural proteins, VP1 to VP4, while P2 and P3 are precursors of the seven nonstructural proteins; 2A, 2B, 2C, 3A, VPg, 3C, and RdRp, respectively.
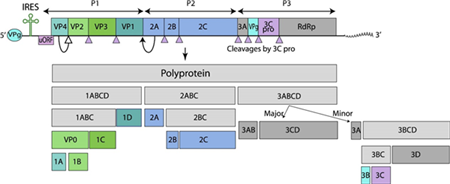
Fig. 2 Genome organization of Coxsackieviruses (viralzone)
Virion Proteins
Coxsackievirus virions consist of four structural proteins—VP1, VP2, VP3, and VP4—along with the RNA genome, and only mature virions are capable of infectivity. Among these, VP1 occupies the majority of the accessible surface of the virus particle and is the most immunogenic and variable protein, playing a key role in stimulating the host's production of neutralizing antibodies. In addition to the mature virions, empty procapsids composed of VP0, VP1, and VP3, as well as other viral intermediates, are generated during virus production in V937 cell culture. VP0 undergoes cleavage into VP2 and VP4 after the encapsidation of the RNA genome, resulting in the formation of infectious, mature virions.
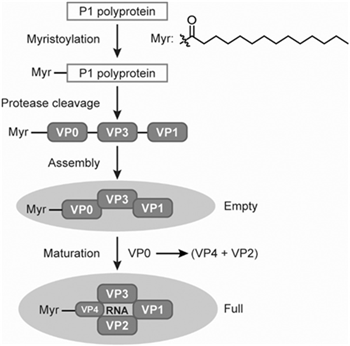
Fig. 3 Coxsackievirus VPs and virus capsids formation. VPs, virion proteins. (Deng et al., 2022)
Protease 2A
The 2A protease of enterovirus is a nonstructural protein that forms hexamers and exhibits both self-cleavage activity and the ability to cleave the eukaryotic translation initiation factor 4G (eIF4G). It plays a crucial role in the processing of the viral polyprotein, mediating the initial cleavage between the P1 and P2 regions. In addition to its autocatalytic activity, 2A protease cleaves eIF4G1, effectively disrupting cellular mRNA translation by inhibiting the cap-dependent translation initiation. Furthermore, the protease targets and cleaves the host antiviral protein IFIH1/MDA5, thereby impairing type-I interferon (IFN) production and inhibiting the establishment of an antiviral state in the host cell.
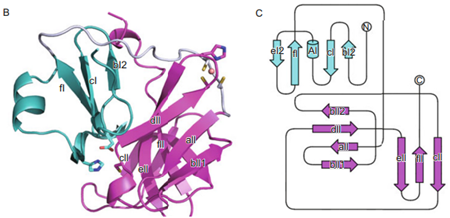
Fig. 4 Crystal structure and topological graph of CVA16A Protease 2A. (Sun et al., 2013)
Protein 2B
Protein 2B of Coxsackievirus plays a critical role in the viral replication cycle, primarily by functioning as a viroporin. It induces autophagy in host cells through its transmembrane hydrophobic regions, facilitating the formation of autophagosomes. The ability of 2B to trigger autophagy is mediated by its hydrophobic motifs, with a specific structural region between these motifs, including a critical valine residue (56V), being essential for this process. Additionally, 2B forms a pore in the host endoplasmic reticulum (ER), resulting in the release of Ca²⁺ into the cytoplasm. The elevated cytoplasmic calcium concentration promotes membrane trafficking and the transport of viral ER-associated proteins to viroplasms, which are sites for viral genome replication. These actions underscore the multifaceted role of 2B in manipulating host cellular processes to support viral replication.
Protein 2C
The 2C proteins of Coxsackievirus are multifunctional components essential for viral replication. Containing conserved SF3 helicase motifs, they act as RNA helicases, unwinding RNA in an NTP-dependent manner, and as RNA chaperones, promoting RNA strand annealing independently of NTPs. Their RNA helicase and NTPase activities are dependent on divalent metal ions. Additionally, 2C proteins induce structural rearrangements of intracellular membranes and are involved in virion morphogenesis and viral RNA encapsidation, likely through interactions with the capsid protein VP3. These findings highlight the critical role of 2C proteins in RNA remodeling, membrane dynamics, and the overall viral lifecycle.
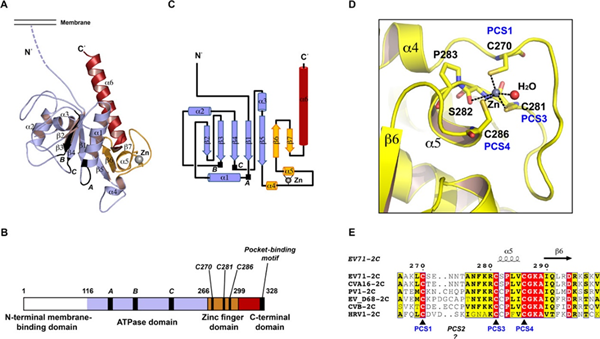
Fig. 5 Overall structure of Protein 2C (Guan et al., 2017)
Protein 3A
Coxsackievirus 3A protein is essential for viral replication and host cell manipulation. It facilitates viral RNA replication by recruiting ACBD3 and PI4KB to replication sites, promoting the formation of membranous structures essential for viral replication. 3A also localizes the viral replication complex to the surface of membranous vesicles and disrupts endoplasmic reticulum-to-Golgi trafficking, likely through interaction with the host protein GBF1, which results in the depletion of MHC, TRAIL, and IFN receptors on the cell surface. Additionally, 3A forms homodimers and suppresses RNA interference (RNAi) by directly interacting with double-stranded RNAs (dsRNAs) and small interfering RNAs (siRNAs). These findings underscore the critical role of 3A in both viral RNA replication and immune evasion.
Protease 3C
Coxsackievirus 3C proteinase (3Cpro) is a critical cysteine protease that plays a central role in viral polyprotein processing, mediating all but one of the proteolytic cleavages required for viral maturation. In addition to its role in viral replication, 3Cpro contributes to the suppression of host cellular processes to facilitate viral propagation. It cleaves host proteins such as EIF5B and PABPC1, leading to the shutdown of host translation. Furthermore, 3Cpro binds and inhibits the host antiviral sensor IFIH1/MDA5, preventing type-I interferon (IFN) production and impairing the establishment of an antiviral state. 3Cpro also cleaves host MAP3K7/TAK1, inhibiting TRAF6-mediated NF-kappa-B activation, and targets NLRP1, triggering its autophagic degradation. These actions underscore 3Cpro’s pivotal role in both viral replication and immune evasion. Due to its essential functions, 3Cpro represents a promising therapeutic target for the treatment of Coxsackievirus and other enteroviral infections, although no specific inhibitors have yet been developed for clinical use.
Host species: Human
Isotype: IgG1
Applications: ELISA, Neutralization, X-ray crystallography
Accession: Q9QF31
Host species: Human
Isotype: IgG1, kappa
Applications: ELISA, Neutralization, X-ray crystallography
Accession: Q9QF31
Host species: Mouse
Isotype: IgG2a
Applications: Block, IHC
Accession: A0A0K2BNC7
Host species: Rabbit
Isotype: IgG
Applications: ELISA, IHC, WB
Accession: USC32119.1
Host species: Rabbit
Isotype: IgG
Applications: ELISA, IHC, WB
Accession: Q65900
Host species: Rabbit
Isotype: IgG
Applications: ELISA, IHC, WB
Accession: Q9QF31
Host species: Rabbit
Isotype: IgG
Applications: ELISA, IHC, WB
Accession: Q9QF31
Host species: Rabbit
Isotype: IgG
Applications: ELISA, IHC, WB
Accession: Q9QF31
Host species: Rabbit
Isotype: IgG
Applications: ELISA, IHC, WB
Accession: Q9QF31
Host species: Rabbit
Isotype: IgG
Applications: ELISA, IHC, WB
Accession: Q9QF31