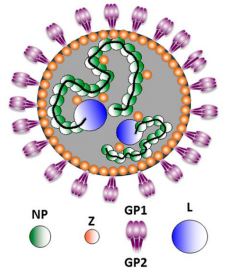
Lassa virus virion structure(Viruses vol. Jul. 2016)
The virions of arenaviruses are spherical in appearance and between 40 and 200 nm in diameter. The surface has a spike structure formed by the envelope glycoprotein GPC (glycoprotein complex). The inner support of the virus envelope is the matrix protein Z (RING finger zinc-binding protein), the nucleocapsid protein NP (nucleocapsid protein)wraps two single-stranded RNA genes, and together with the RNA-dependent RNA polymerase L protein (RNA-dependent RNA polymerase) constitutes thenucleocapsid protein (ribonucleoprotein) of the virus.
Lassa virus is a double-segmented negative-strand RNA virus. The genome includes an S segment of about 3500 nt and an L segment of 7200 nt. Each genome encodes two proteins, separated by a non-coding intergenic region IGR (intergenic region). The S segment encodes the nucleoprotein NP (63 kDa) and the glycoprotein precursor GPC (75 kDa), and the L segment encodes the RNA polymerase L protein (200 kDa) and the matrix protein Z.
Arenaviruses are surrounded by a lipid bilayer containing the post-translationally processed viral glycoprotein involved in receptor binding (GP1) and viral cell entry (GP2). Underneath the lipid bilayer is a protein layer composed of the Z protein, which plays a major role in viral assembly and budding, and is the arenavirus counterpart of the matrix protein present in other enveloped negative-stranded (NS) RNA viruses. The core of the virus is made of a viral ribonucleoprotein (vRNP) complex, composed of the viral genome segments encapsidated by the viral NP . Incorporation of the vRNPs into newly nascent virions is mediated by NP-Z interaction. Associated with the vRNPs is the L polymerase protein that,together with NP , are the minimal components for viral genome replication and gene transcription.
Pre-glycoprotein polyprotein GP complex(P08669)
Function
Glycoprotein G1
Interacts with the host receptor (By similarity).
Mediates virus attachment to host receptor alpha-dystroglycan DAG1. This attachment induces virion internalization predominantly through clathrin- and caveolin-independent endocytosis
Glycoprotein G2
Class I viral fusion protein that directs fusion of viral and host endosomal membranes, leading to delivery of the nucleocapsid into the cytoplasm. Membrane fusion is mediated by irreversible conformational changes induced upon acidification in the endosome.
Stable signal peptide (SSP)
Cleaved and functions as a signal peptide. In addition, it is also retained as the third component of the GP complex. The SSP is required for efficient glycoprotein expression, post-translational maturation cleavage of GP1 and GP2, glycoprotein transport to the cell surface plasma membrane, formation of infectious virus particles, and acid pH-dependent glycoprotein-mediated cell fusion.
Nucleoprotein(P13699)
Function
Encapsidates the genome, protecting it from nucleases. The encapsidated genomic RNA is termed the nucleocapsid (NC). Serves as template for viral transcription and replication. The increased presence of protein N in host cell does not seem to trigger the switch from transcription to replication as observed in other negative strain RNA viruses. Through the interaction with host IKBKE, strongly inhibits the phosphorylation and nuclear translocation of host IRF3, a protein involved in interferon activation pathway, leading to the inhibition of interferon-beta and IRF3-dependent promoters activation. Encodes also a functional 3'-5' exoribonuclease that degrades preferentially dsRNA substrates and thereby participates in the suppression of interferon induction.
RING finger protein Z(O73557)
Function
Plays a crucial role in virion assembly and budding. Expressed late in the virus life cycle, it acts as an inhibitor of viral transcription and RNA synthesis by interacting with the viral polymerase L. Presumably recruits the NP encapsidated genome to cellular membranes at budding sites via direct interaction with NP. Plays critical roles in the final steps of viral release by interacting with host TSG101, a member of the vacuolar protein-sorting pathway and using other cellular host proteins involved in vesicle formation pathway. The budding of the virus progeny occurs after association of protein Z with the viral glycoprotein complex SSP-GP1-GP2 at the cell periphery, step that requires myristoylation of protein Z. Also selectively represses protein production by associating with host eIF4E.
RNA-directed RNA polymerase L(O09705)
Function
RNA-dependent RNA polymerase which is responsible for replication and transcription of the viral RNA genome. During transcription, synthesizes 4 subgenomic RNAs, and assures their capping by a cap-snatching mechanism, in which cellular capped pre-mRNAs are used to generate primers for viral transcription. The 3'-end of subgenomic mRNAs molecules are heterogeneous and not polyadenylated. The replicase function is to direct synthesis of antigenomic and genomic RNA which are encapsidated and non capped. As a consequence of the use of the same enzyme for both transcription and replication, these mechanisms need to be well coordinated. These processes may be regulated by proteins N and Z in a dose-dependent manner.
References
Applications: ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Expression system: Mammalian Cells
Accession: Q00G17
Protein length: Met1-Tyr459
Host species: Human
Isotype: IgG1, kappa
Applications: ELISA, Neutralization
Accession: P08669
Host species: Rabbit
Isotype: IgG
Applications: ELISA, IHC, WB
Accession: P08669
Host species: Rabbit
Isotype: IgG
Applications: ELISA, IHC, WB
Accession: P08669
Host species: Rabbit
Isotype: IgG
Applications: ELISA, IHC, WB
Accession: O73557
Applications: ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Expression system: E. coli
Accession: P08669
Protein length: Gly260-Asp432
Applications: ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Expression system: E. coli
Accession: P08669
Protein length: Thr59-Leu259
Applications: ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Expression system: E. coli
Accession: O73557
Protein length: Gly2-Pro99
Host species: Human
Isotype: IgG1-kappa
Applications: Research Grade Biosimilar
Expression system: Mammalian Cells
Host species: Human
Isotype: IgG1, kappa
Applications: ELISA, Neutralization
Expression system: Mammalian Cells