Catalog No.
YXX06201
Expression system
E. coli
Species
Escherichia coli
Protein length
Met1-Lys784
Predicted molecular weight
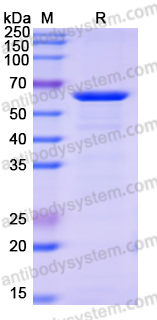
89.60 kDa
Nature
Recombinant
Endotoxin level
Please contact with the lab for this information.
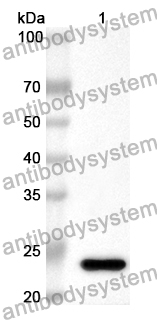
Purity
>90% as determined by SDS-PAGE.
Accession
P0A9M1
Applications
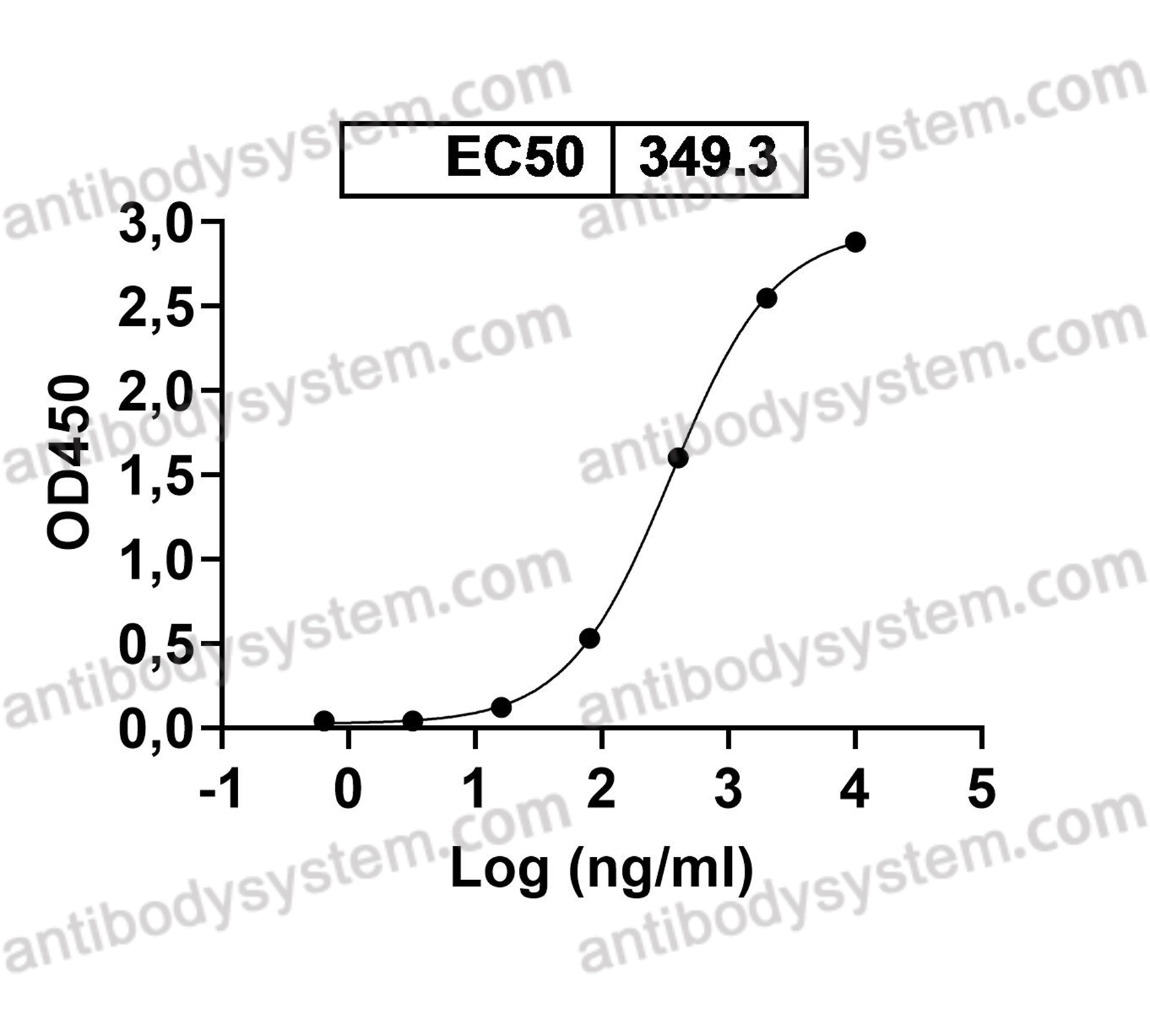
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Reconstitution
Reconstitute in sterile water for a stock solution. A copy of datasheet will be provided with the products, please refer to it for details.
Shipping
In general, proteins are provided as lyophilized powder/frozen liquid. They are shipped out with dry ice/blue ice unless customers require otherwise.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8°C for frequent use. Store at -20 to -80°C for twelve months from the date of receipt.
Alternative Names
Lon protease, ATP-dependent protease La, lon, c0555
Transcriptomic and proteomic ramifications of segmental amplification in Escherichia coli., PMID:40372434
The protein degradation system encoded by hslUV (ClpYQ) is dispensable for the virulence of Haemophilus ducreyi in human volunteers., PMID:40208051
The crystal structure of the toxin EspC from enteropathogenic Escherichia coli reveals the mechanism that governs host cell entry and cytotoxicity., PMID:40164999
Structural basis for the allosteric activation of Lon by the heat shock protein LarA., PMID:40044692
Multitarget mechanism of MYC inhibition by the bacterial lon protease in disease., PMID:40000737
Bacteria encode post-mortem protein catabolism that enables altruistic nutrient recycling., PMID:39948360
Expression of Multiple Copies of the Lon Protease Gene Resulted in Increased Antibiotic Production, Osmotic and UV Stress Resistance in Streptomyces coelicolor A3(2)., PMID:39690306
Allosteric modulation of the Lon protease via ssDNA binding and local charge changes., PMID:39542252
Use of transcriptomics and genomics to assess the effect of disinfectant exposure on the survival and resistance of Escherichia coli O157:H7, a human pathogen., PMID:39507346
Identification and genetic dissection of convergent persister cell states., PMID:39506104
Membrane alteration, anti-virulence properties and metabolomic perturbation of a chionodracine-derived antimicrobial peptide, KHS-Cnd, on two bacteria models., PMID:39426570
A type II toxin-antitoxin system, ECs3274-ECs3275, in enterohemorrhagic Escherichia coli O157., PMID:39424600
Uncovering the effects of non-lethal oxidative stress on replication initiation in Escherichia coli., PMID:39389326
Filament formation activates protease and ring nuclease activities of CRISPR Lon-SAVED., PMID:39362215
BpfD Is a c-di-GMP Effector Protein Playing a Key Role for Pellicle Biosynthesis in Shewanella oneidensis., PMID:39273643
Impact of a random TN5 mutation on endoglucanase secretion in ruminal cellulolytic Escherichia coli., PMID:39260626
The importance of the location of the N-terminus in successful protein folding in vivo and in vitro., PMID:39145938
Protein degradation by a component of the chaperonin-linked protease ClpP., PMID:38965067
Suppression of the Escherichia coli rnpA49 conditionally lethal phenotype by different compensatory mutations., PMID:38688559
Divergent Roles of Escherichia Coli Encoded Lon Protease in Imparting Resistance to Uncouplers of Oxidative Phosphorylation: Roles of marA, rob, soxS and acrB., PMID:38372817
Expression and Purification of Recombinant Human Mitochondrial RNA Polymerase (POLRMT) and the Initiation Factors TFAM and TFB2M., PMID:38094251
The heat shock protein LarA activates the Lon protease in response to proteotoxic stress., PMID:37993443
A 5+1 assemble-to-activate mechanism of the Lon proteolytic machine., PMID:37957149
Protein-Protein Interaction: Tandem Affinity Purification in Bacteria., PMID:37930536
Lon degrades stable substrates slowly but with enhanced processivity, redefining the attributes of a successful AAA+ protease., PMID:37660294
Advancing evolution: Bacteria break down gene silencer to express horizontally acquired genes., PMID:37533411
Affinity isolation and biochemical characterization of N-degron ligands using the N-recognin, ClpS., PMID:37532398
Identification and Characterization of an HtrA Sheddase Produced by Coxiella burnetii., PMID:37446087
Validation of Lon Gene Disruption using Linear DNA Cassette by Crelox Mechanism in E. coli Strains: To Achieve Better Solubility of Putrescine Monooxygenase., PMID:37188228
Yttrium Oxide Nanoparticle-Loaded, Self-Assembled Peptide Gel with Antibacterial, Anti-Inflammatory, and Proangiogenic Properties for Wound Healing., PMID:37097124
Biodegradation of Pine Processionary Caterpillar Silk Is Mediated by Elastase- and Subtilisin-like Proteases., PMID:36499578
Efficiency of CRISPR-Cas9 genetic engineering in Escherichia coli BL21 is impaired by lack of Lon protease., PMID:36470413
Degradation of gene silencer is essential for expression of foreign genes and bacterial colonization of the mammalian gut., PMID:36161931
ATP hydrolysis tunes specificity of a AAA+ protease., PMID:36130509
Kinetics of Bovine leukemia virus aspartic protease reveals its dimerization and conformational change., PMID:35867649
Biogeographic Variation and Functional Pathways of the Gut Microbiota in Celiac Disease., PMID:35810781
Shotgun Proteomics Revealed Preferential Degradation of Misfolded In Vivo Obligate GroE Substrates by Lon Protease in Escherichia coli., PMID:35744894
Catestatin selects for colonization of antimicrobial-resistant gut bacterial communities., PMID:35440728
Expression of the First Recombinant Anti-Tumoral Snake Venom Kunitz-Type Serine Protease Inhibitor., PMID:35324668
In silico Investigation of Lon Protease as a Promising Therapeutic Target., PMID:35042266
lon Deletion Impairs Persister Cell Resuscitation in Escherichia coli., PMID:35038905
ParD Antitoxin Hotspot Alters a Disorder-to-Order Transition upon Binding to Its Cognate ParE Toxin, Lessening Its Interaction Affinity and Increasing Its Protease Degradation Kinetics., PMID:34914378
Enhancement of membrane vesicle production by disrupting the degP gene in Meiothermus ruber H328., PMID:34910268
Production, purification and characterization of a double-tagged TEV protease., PMID:34798273
Genomic analysis of shiga toxin-containing Escherichia coli O157:H7 isolated from Argentinean cattle., PMID:34710106
Essential roles of Lon protease in the morpho-physiological traits of the rice pathogen Burkholderia glumae., PMID:34525127
Low Cytoplasmic Magnesium Increases the Specificity of the Lon and ClpAP Proteases., PMID:33941609
Towards the Enhancement of Essential Oil Components' Antimicrobial Activity Using New Zein Protein-Gated Mesoporous Silica Microdevices., PMID:33917595
Expression in Escherichia coli, purification and kinetic characterization of LAPLm, a Leishmania major M17-aminopeptidase., PMID:33775769
DNA damage-signaling, homologous recombination and genetic mutation induced by 5-azacytidine and DNA-protein crosslinks in Escherichia coli., PMID:33743507