Catalog No.
KDA42301
Description
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Recombinant Human VEGFA has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and Faricimab will be captured by immobilized Human VEGFA. After washing away any unbound substances, a biotin-labeled Human ANGPT2 is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Faricimab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Faricimab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
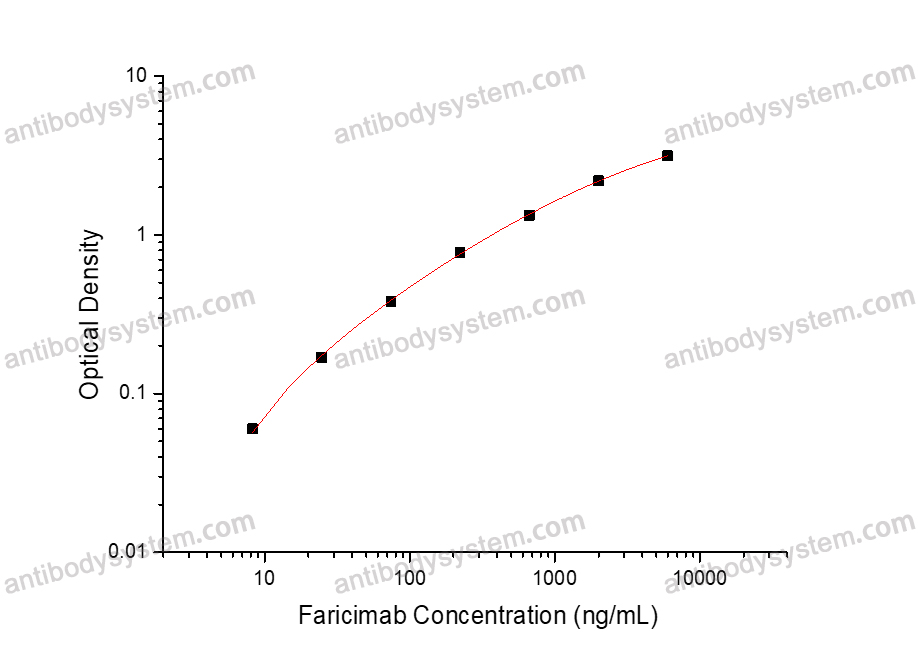
Range
8.23 - 6,000 ng/mL
Sensitivity
4.51 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
1657.6
|
208.9
|
19.9
|
1667.7
|
195.3
|
20.8
|
|
Standard deviation
|
124.7
|
12.4
|
1.5
|
192.6
|
17.1
|
3.0
|
|
CV (%)
|
7.5
|
5.9
|
7.6
|
11.6
|
8.7
|
14.3
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
RG7716, RO6867461, CAS: 1607793-29-2
Background
Faricimab: an investigational agent targeting the Tie-2/angiopoietin pathway and VEGF-A for the treatment of retinal diseases
Quantification of Hyperreflective Foci in Age-related Macular Degeneration by Polarization-Sensitive OCT., PMID:40502292
Effect of Faricimab versus Aflibercept on Hyperreflective Foci in Patients with Diabetic Macular Edema from the YOSEMITE/RHINE Trials., PMID:40496217
Intraocular Inflammation Following Faricimab Intravitreal Injection Treated With Sub-tenon Triamcinolone Acetonide Injection., PMID:40491639
Anti-VEGFs for Diabetic Macular Oedema: Analysis of Efficacy, Safety, and Cost of More Durable Therapies from a Dutch Societal Perspective., PMID:40481910
Retinal Vasculitis after Intravitreal Faricimab Injection., PMID:40478546
Early Anatomical and Functional Outcomes of Faricimab in Recalcitrant Neovascular Age-Related Macular Degeneration: A Retrospective Real-World Study in an East-Asian Population., PMID:40465138
Author Correction: Effects of switching from intravitreal injection of aflibercept to faricimab on ocular blood flow in patients with diabetic macular edema., PMID:40456888
Association between intravitreal anti-vascular endothelial growth factor agents and hypertension: a meta-analysis., PMID:40456283
Real-Life Treatment Intervals and Morphological Outcomes Following the Switch to Faricimab Therapy in Neovascular Age-Related Macular Degeneration., PMID:40423060
Effectiveness and safety of anti-vascular endothelial growth factor therapies for macular edema in retinal vein occlusion: A systematic review and network meta-analysis of randomized controlled trials., PMID:40419166
Successful Response to Intravitreal Faricimab Injections in a Case of Neovascular Age-Related Macular Degeneration in Vitrectomized Eyes., PMID:40400877
[Faricimab in previously treated neovascular age-related macular degeneration : Study design of the prospective noninterventional study PASSENGER]., PMID:40397174
Twelve-Month Outcomes of Faricimab for Patients with Sub-Optimally Responsive Diabetic Macular Oedema: A Retrospective Single-Centre Study., PMID:40396158
Early Outcomes After Initiation of Faricimab in Patients With Diabetic Macular Edema., PMID:40371971
Early Outcomes After Initiation of Faricimab for Neovascular Age-Related Macular Degeneration., PMID:40371970
Faricimab Reverts VEGF-A165-Induced Impairment of the Barrier Formed by Retinal Endothelial Cells., PMID:40362554
Silicone oil microdroplets from syringes with intravitreal anti-vascular endothelial growth factor and complement inhibitor injections., PMID:40359347
Short-term real-world outcomes of diabetic macular edema treated with intravitreal faricimab., PMID:40359224
Real-World Data on Morphological and Functional Responses After Switching to Faricimab in Recalcitrant, Chronic Diabetic Macular Edema., PMID:40355730
Outcomes by Faricimab Treatment Interval at Week 48 of TENAYA-LUCERNE Phase 3 Trials in Neovascular Age-Related Macular Degeneration., PMID:40349982
Two-year outcomes of treat-and-extend regimen with intravitreal faricimab for treatment-naïve neovascular age-related macular degeneration., PMID:40347367
Improved functional and morphological outcomes with faricimab in nAMD eyes with poor response to prior intravitreal anti-VEGF therapy., PMID:40338383
One-year functional and structural results of faricimab for treatment-naïve neovascular age related macular degeneration: An OCT angiography study., PMID:40329091
Tailored Anti-VEGF Therapy with New Generation Optimizations (TANGO) Treatment Regimen for Neovascular Age-Related Macular Degeneration: Rationale, Design, and Simulation Study., PMID:40321164
Short-term comparison of switching to faricimab from other anti-VEGF agents in neovascular age-related macular degeneration patients: A retrospective study., PMID:40295235
Intraocular Inflammation, Safety Events, and Outcomes After Intravitreal Injection of Ranibizumab, Aflibercept, Brolucizumab, Abicipar Pegol, and Faricimab for nAMD., PMID:40271423
Bilateral Sterile Granulomatous Uveitis Caused by Intravitreal Injections of Faricimab., PMID:40271422
[Intravitreal Faricimab Injections for Coats Disease: a Case Report]., PMID:40267947
Early Outcomes of Faricimab Treatment for Diabetic Macular Edema in Patients Previously Treated With Anti-VEGF Therapy., PMID:40258191
Real-World Evidence for Faricimab in Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema: A Scoping Review., PMID:40242371
Comparative efficacy of intravitreal anti-VEGF therapy for neovascular age-related macular degeneration: A systematic review with network meta-analysis., PMID:40241463
Intravitreal injection of faricimab to treat macular and retinovascular diseases in Nigerians: Early real-world experience., PMID:40233664
Safety profile of faricimab: a multi-source pharmacovigilance analysis using FAERS and JADER., PMID:40221797
Switching to Faricimab in Therapy-Resistant Macular Edema Due to Retinal Vein Occlusion: Initial Real-World Efficacy Outcomes., PMID:40217902
Deep learning assisted analysis of biomarker changes in refractory neovascular AMD after switch to faricimab., PMID:40217505
Efficacy of Aflibercept (8 mg) for Diabetic Macular Edema in Vitrectomized Eyes Refractory to the Other Anti-VEGF Drug Therapies: A Report of Three Cases., PMID:40206271
Occlusive Retinal Vasculitis After Aflibercept 8mg Injection for Wet Macular Degeneration., PMID:40184546
Adverse event reporting of faricimab: a disproportionality analysis of FDA adverse event reporting system (FAERS) database., PMID:40144657
One-Year Outcomes after Switching to Faricimab in Eyes with Pretreated Neovascular Age-Related Macular Degeneration: A Swiss Retina Research Network Report., PMID:40139459
Efficacy of treatment with faricimab for patients with refractory nAMD., PMID:40134284
Deep-Learning-Assisted Analysis of Early Biomarker Changes in Treatment-Naïve Patients with Neovascular AMD Under Intravitreal Faricimab., PMID:40133689
Surprising complete regression of a choroidal neovascular membrane with faricimab., PMID:40120750
Faricimab Treat-and-Extend Dosing for Macular Edema Due to Retinal Vein Occlusion: 72-Week Results from the BALATON and COMINO Trials., PMID:40107501
Recurrent uveitic macular edema managed with intravitreal faricimab injection., PMID:40080237
Cost-effectiveness analysis of bispecific antibody faricimab for treatment of neovascular age-related macular degeneration and diabetic macular edema in Japan., PMID:40078048
Comparison of intraocular pressure changes in Japanese patients with neovascular age-related macular degeneration treated with aflibercept or faricimab., PMID:40072815
Economic impact of a faricimab vial-sharing protocol in age-related macular degeneration and diabetic macular oedema patients., PMID:40071859
Real-world experience of intravitreal faricimab injection in previously treated neovascular age-related macular degeneration eyes: a case series., PMID:40065260
Cost-Effectiveness and Budget Impact Analysis of the Use of Faricimab in Diabetic Macular Edema and Neovascular Age-Related Macular Degeneration in Colombia., PMID:40051780