Catalog No.
KDH28808
Description
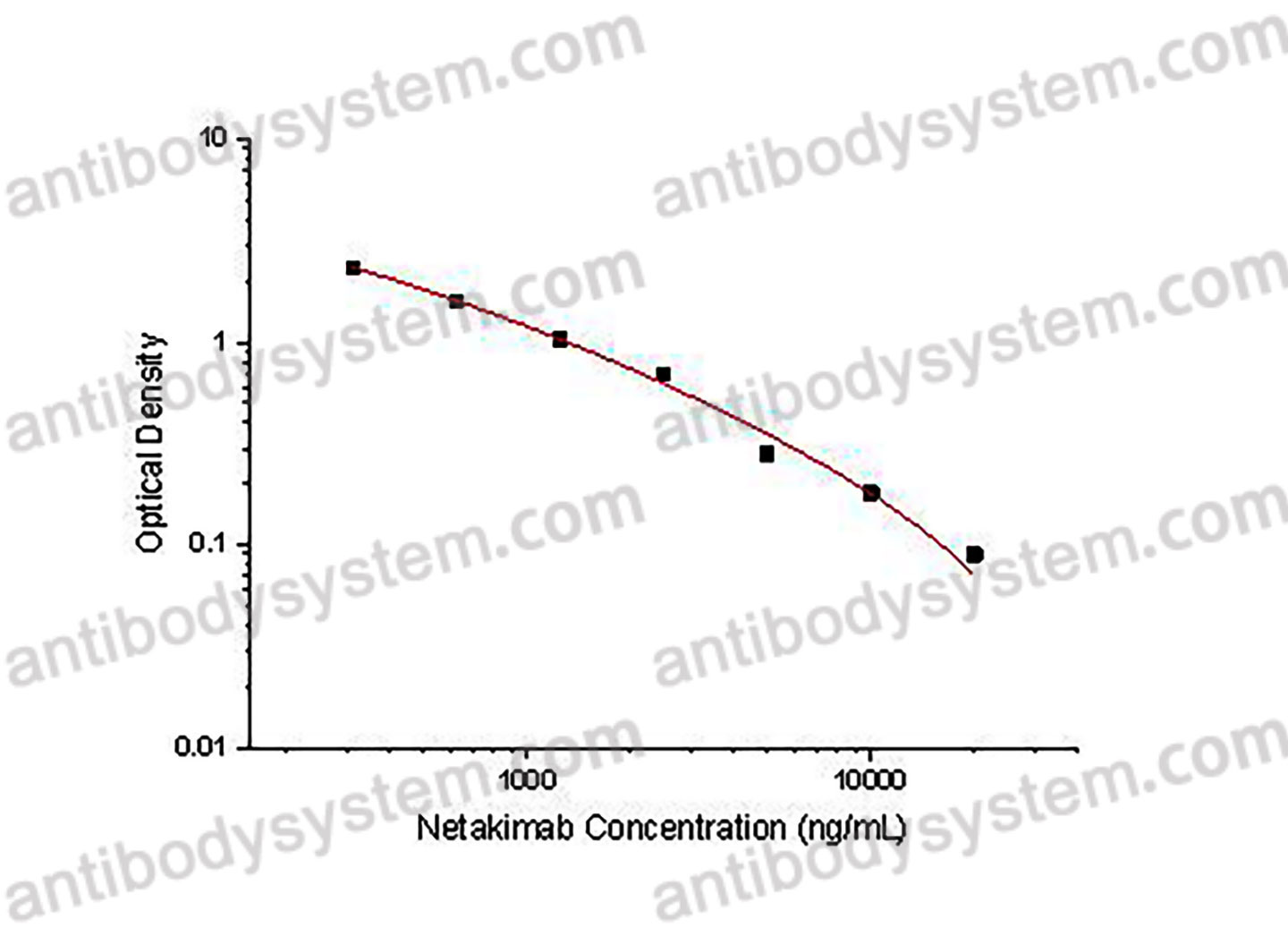
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human IL17A has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Netakimab in the sample competitively binds to the pre-coated protein with biotin-labeled Netakimab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Netakimab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Netakimab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
312.5- 20,000 ng/mL
Sensitivity
216.52 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
8382.1
|
1955.7
|
607.5
|
10240.5
|
2317.0
|
586.7
|
|
Standard deviation
|
519.0
|
93.0
|
63.9
|
478.9
|
111.1
|
98.0
|
|
CV (%)
|
6.2
|
4.8
|
10.5
|
4.7
|
4.8
|
16.7
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
AG1-25, CAS: 1796570-08-5
Efficacy and Safety of Interleukin-17 and Janus Kinase Inhibitors in Ankylosing Spondylitis: A Systematic Review and Network Meta-Analysis., PMID:40010328
Next-Generation Anti-IL-17 Agents for Psoriatic Disease: A Pipeline Review., PMID:39982633
Interleukin-17: A pleiotropic cytokine implicated in inflammatory, infectious, and malignant disorders., PMID:39875232
Real-World Retention Rate, Effectiveness, and Safety of Netakimab in the Treatment of Patients with Ankylosing Spondylitis: First Year Results of the LIBRA Post-Registration Safety Study., PMID:39196530
Course of Uveitis in Patients with Ankylosing Spondylitis during the Interleukin17 Inhibitors Therapy., PMID:38861150
[Current treatment for spondyloarthritis: focus on netakimab. A review]., PMID:38829817
[Real-world experience with netakimab in the treatment of spondyloarthritis]., PMID:38785057
NEUROPROTECTIVE AND ANTIOXIDANT POTENTIAL OF MONTELUKAST-ACETYLCYSTEINE COMBINATION THERAPY FOR BRAIN PROTECTION IN PATIENTS WITH COVID-19 INDUCED PNEUMONIA., PMID:37042600
Two Epitope Regions Revealed in the Complex of IL-17A and Anti-IL-17A VHH Domain., PMID:36499233
Biological treatment for erythrodermic psoriasis., PMID:36154361
Response to netakimab in radiographic axial spondyloarthritis patients with different baseline C-reactive protein, sacroiliitis evaluated by MRI and peripheral joint involvement status: a post-hoc analysis of the ASTERA study., PMID:36062743
Tocilizumab, netakimab, and baricitinib in patients with mild-to-moderate COVID-19: An observational study., PMID:36001576
Immune-related adverse events (irAEs) in ankylosing spondylitis (AS) patients treated with interleukin (IL)-17 inhibitors: a systematic review and meta-analysis., PMID:35188599
Interleukin 17 antagonist netakimab is effective and safe in the new coronavirus infection (COVID-19)., PMID:34346869
Latest Advances for the Treatment of Chronic Plaque Psoriasis with Biologics and Oral Small Molecules., PMID:34239295
Anti-IL-17 monoclonal antibodies in hospitalized patients with severe COVID-19: A pilot study., PMID:34237556
Efficacy and Safety of Netakimab, A Novel Anti-IL-17 Monoclonal Antibody, in Patients with Moderate to Severe Plaque Psoriasis. Results of A 54-Week Randomized Double-Blind Placebo-Controlled PLANETA Clinical Trial., PMID:34060012
Anti-IL-17 Agents in the Treatment of Axial Spondyloarthritis., PMID:33977094
Efficacy and safety of interleukin-17 inhibitors in the treatment of chronic rheumatic diseases: A combined and updated meta-analysis., PMID:33768576
Efficacy and safety of interleukin-17A inhibitors in patients with ankylosing spondylitis: a systematic review and meta-analysis of randomized controlled trials., PMID:33432451
Emerging systemic drugs in the treatment of plaque psoriasis., PMID:32192366
State of the art and pharmacological pipeline of biologics for chronic plaque psoriasis., PMID:31212119
Primary efficacy of netakimab, a novel interleukin-17 inhibitor, in the treatment of active ankylosing spondylitis in adults., PMID:31025924
IL-17 inhibition in axial spondyloarthritis: current and future perspectives., PMID:30957574