Catalog No.
KDB86903
Description
PRINCIPLE OF THE ASSAY
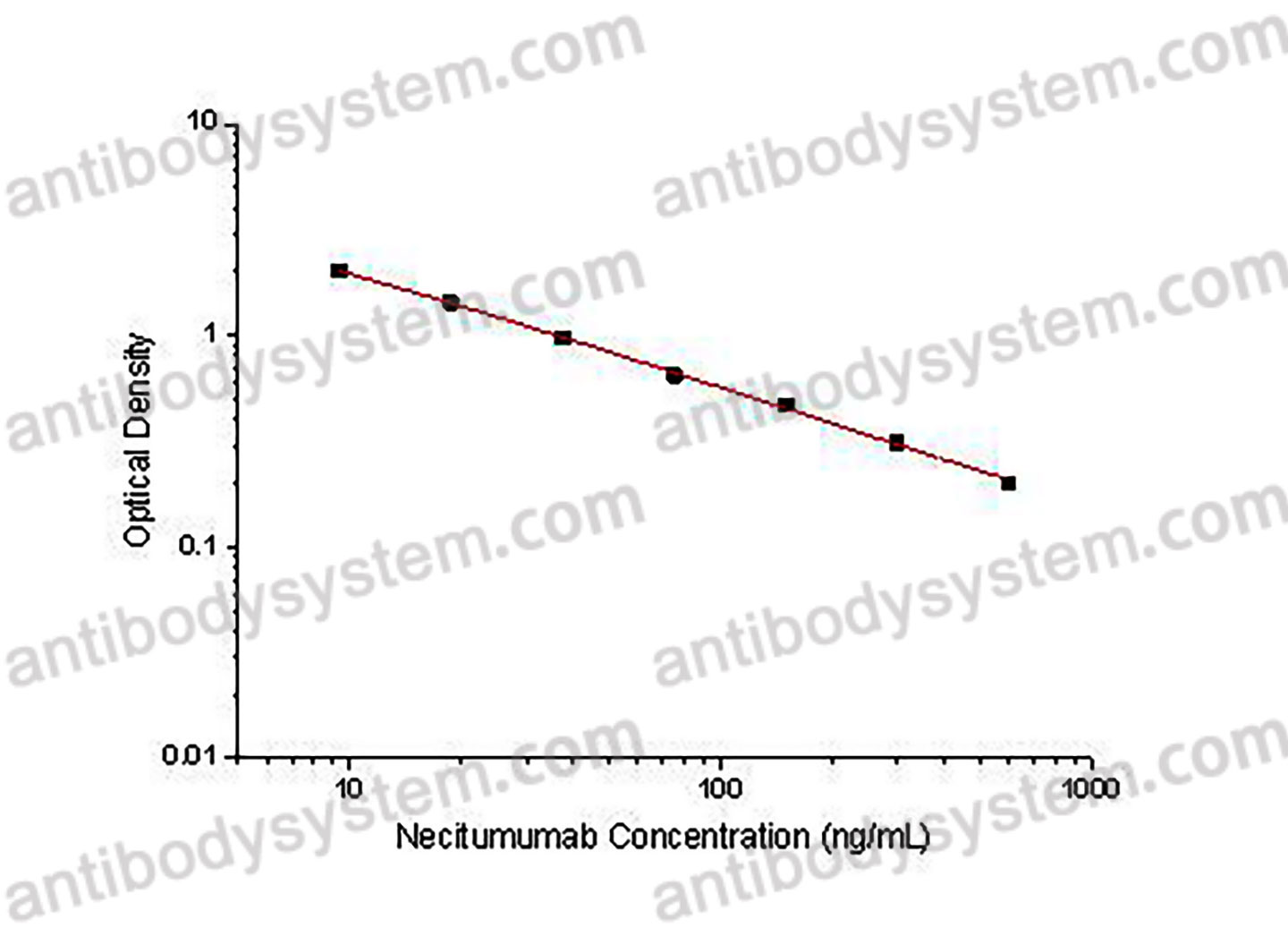
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human EGFR has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Necitumumab in the sample competitively binds to the pre-coated protein with biotin-labeled Necitumumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Necitumumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Necitumumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
9.38 - 600 ng/mL
Sensitivity
6.05 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
392.4
|
71.0
|
24.2
|
231.4
|
65.8
|
23.4
|
|
Standard deviation
|
61.0
|
9.5
|
3.6
|
44.5
|
11.9
|
4.5
|
|
CV (%)
|
15.5
|
13.4
|
15.1
|
19.2
|
18.1
|
19.2
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
11F8, IMC-11F8, LY3012211, CAS: 906805-06-9
Background
Necitumumab (IMC-11F8, LY3012211) is a second-generation fully human immunoglobulin (Ig) G1 kappa isotype monoclonal antibody (mAb) that acts as an antagonist to direct against the extracellular region of epidermal growth factor receptor (EGFR). It was developed by Eli Lilly & Co (Indianapolis, IN, USA) and produced in genetically engineered mammalian NS0 cells. It was first approved by the U.S. Food and Drug Administration (FDA) under the brand name Portrazza in 2015, for use in combination with cisplatin and gemcitabine as a first-line treatment for metastatic squamous non-small cell lung cancer (NSCLC). After that, it has gained the approval for marketing in the Europe Union and Japan respectively in 2016 and 2017. It is not indicated for treatment of non-squamous NSCLC. Currently, there are several ongoing clinical trials investigating necitumumab in the treatment of NSCLC in several di铿€erent settings. It is currently being studied in combination with pre-existing agents, such as osimertinib, pembrolizumab, nabpaclitaxel, and carboplatin, as well as with new agents under investigation, abemaciclib and AZD9291. Necitumumab is currently being evaluated in the second-line setting following treatment failure or progression with front-line EGFR tyrosine kinase inhibitors (TKIs) and platinum-based chemotherapy.
ORCHARD: Osimertinib Plus Necitumumab in Patients With Epidermal Growth Factor Receptor-Mutated Advanced Non-Small Cell Lung Cancer With a Secondary Epidermal Growth Factor Receptor Alteration Whose Disease Had Progressed on First-Line Osimertinib., PMID:40466026
Skin disorder within 30 days is a favorable prognostic factor in patients with lung squamous cell carcinoma treated with necitumumab plus gemcitabine and cisplatin: a sub-analysis of the NINJA study., PMID:40093977
A Case of EGFR-mutant Squamous Cell Lung Cancer Treated With Necitumumab Combination Therapy., PMID:40010994
Clinical impact of hypomagnesemia induced by necitumumab plus cisplatin and gemcitabine treatment in patients with advanced lung squamous cell carcinoma: a subanalysis of the NINJA study., PMID:39957806
Afatinib and Necitumumab in EGFR-Mutant NSCLC with Acquired Resistance to Tyrosine Kinase Inhibitors., PMID:39866193
A Case of Advanced Biliary Tract Cancer With EGFR Amplification That Responded to Necitumumab., PMID:39540676
Transforming Lung Cancer Management: A Promising Case Study of Immune Checkpoint Inhibitor Success Following a Multidisciplinary Approach., PMID:39410563
Effects of N361 Glycosylation on Epidermal Growth Factor Receptor Biological Function., PMID:39071333
Enhancing Neutrophil Cytotoxicity of a Panel of Clinical EGFR Antibodies by Fc Engineering to IgA3.0., PMID:38958494
Association between skin toxicity and efficacy of necitumumab in squamous non-small-cell lung cancer: a pooled analysis of two randomized clinical trials-SQUIRE and JFCM., PMID:38520847
[Enhancement of Antitumor Effect by the Combined Use of Anti-EGFR Antibody(Necitumumab)and PD-1 Inhibitor]., PMID:38247069
Multicenter, Retrospective Study to Evaluate Necitumumab Plus Cisplatin and Gemcitabine After Immune Checkpoint Inhibitors in Advanced Squamous Cell Lung Cancer in Japan: The NINJA Study., PMID:38046378
A Phase I/II Study of Necitumumab Plus Pembrolizumab, Nab-Paclitaxel, and Carboplatin for Previously Untreated Advanced Squamous Non-Small Cell Lung Cancer Study: (NEJ048A/NEXUS)., PMID:36849264
Necitumumab plus gemcitabine and cisplatin in previously treated lung squamous cell carcinoma., PMID:36331673
Acquired perforating dermatosis induced by necitumumab., PMID:35686644
Treatment Sequencing Strategies in Lung Cancer., PMID:35599008
Application of Approved Cisplatin Derivatives in Combination Therapy against Different Cancer Diseases., PMID:35458666
Objective Quantitation of EGFR Protein Levels using Quantitative Dot Blot Method for the Prognosis of Gastric Cancer Patients., PMID:35079437
Accurate determination of epitope for antibodies with unknown 3D structures., PMID:34432559
Lung squamous cell carcinoma with severe hypomagnesemia due to cisplatin plus gemcitabine in combination with necitumumab therapy: A case report., PMID:34061460
EGFR targeting for cancer therapy: Pharmacology and immunoconjugates with drugs and nanoparticles., PMID:33188892
Necitumumab plus platinum-based chemotherapy versus chemotherapy alone as first-line treatment for stage IV non-small cell lung cancer: a meta-analysis based on randomized controlled trials., PMID:32954751
The Latest Battles Between EGFR Monoclonal Antibodies and Resistant Tumor Cells., PMID:32793499
Efficacy and safety of necitumumab and pembrolizumab combination therapy in patients with Stage IV non-small cell lung cancer., PMID:32114283
Effects of adding necitumumab to first-line chemotherapy in patients with stage IV non-small-cell lung cancer: Meta-analysis., PMID:31822198
A phase 1b study of necitumumab in combination with abemaciclib in patients with stage IV non-small cell lung cancer., PMID:31586771
A phase II study of nab-paclitaxel and carboplatin chemotherapy plus necitumumab in the first-line treatment of patients with stage IV squamous non-small cell lung cancer., PMID:31445354
The effects of somatic mutations on EGFR interaction with anti-EGFR monoclonal antibodies: Implication for acquired resistance., PMID:31228284
Corrigendum to "Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line treatment for stage IV squamous non-small cell lung cancer: A phase 1b and randomized, open-label, multicenter, phase 2 trial in Japan" [Lung Cancer, 129 (March) (2019) 55-62]., PMID:31014853
Most clinical anti-EGFR antibodies do not neutralize both wtEGFR and EGFRvIII activation in glioma., PMID:31002307
Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line treatment for stage IV squamous non-small cell lung cancer: A phase 1b and randomized, open-label, multicenter, phase 2 trial in Japan., PMID:30797492
First-line treatment of patients with advanced or metastatic squamous non-small cell lung cancer: systematic review and network meta-analysis., PMID:30746213
The people behind the papers - Elena Popa and Abigail Tucker., PMID:30737241
Necitumumab for the treatment of advanced non-small-cell lung cancer., PMID:30501503
Immune Effector Functions of Human IgG2 Antibodies against EGFR., PMID:30282813
Necitumumab in the treatment of non-small-cell lung cancer: clinical controversies., PMID:30075697
Necitumumab: a new option for first-line treatment of squamous cell lung cancer., PMID:30025476
Algorithm for the treatment of advanced or metastatic squamous non-small-cell lung cancer: an evidence-based overview., PMID:29910650
Predictive biomarkers for response to EGFR-directed monoclonal antibodies for advanced squamous cell lung cancer., PMID:29905778
Breakthroughs in the treatment of advanced squamous-cell NSCLC: not the neglected sibling anymore?, PMID:29862232
The Budget Impact of Including Necitumumab on the Formulary for First-Line Treatment of Metastatic Squamous Non-Small Cell Lung Cancer: U.S. Commercial Payer and Medicare Perspectives., PMID:29799326
Venous thromboembolism with EGFR monoclonal antibody necitumumab in stage IV non-small cell lung cancer: A retrospective cohort analysis., PMID:29787943
A retrospective examination of the US Food and Drug Administration's clinical pharmacology reviews of oncology biologics for potential use of therapeutic drug monitoring., PMID:29343970
Current and Emergent Therapy Options for Advanced Squamous Cell Lung Cancer., PMID:29175116
Molecular Basis for Necitumumab Inhibition of EGFR Variants Associated with Acquired Cetuximab Resistance., PMID:29158469
EGFR Gene Copy Number by FISH May Predict Outcome of Necitumumab in Squamous Lung Carcinomas: Analysis from the SQUIRE Study., PMID:29158193
Efficacy and Safety of Necitumumab Continuation Therapy in the Phase III SQUIRE Study of Patients With Stage IV Squamous Non-Small-Cell Lung Cancer., PMID:29158123
EGFR immunohistochemistry as biomarker for antibody-based therapy of squamous NSCLC - Experience from the first ring trial of the German Quality Assurance Initiative for Pathology (QuIP®)., PMID:29108919
Meta-analysis of first-line therapies with maintenance regimens for advanced non-small-cell lung cancer (NSCLC) in molecularly and clinically selected populations., PMID:28675660