Catalog No.
YVV00802
Expression system
E. coli
Species
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Protein length
Met1-Ala416
Predicted molecular weight
48.57 kDa
Nature
Recombinant
Endotoxin level
Please contact with the lab for this information.
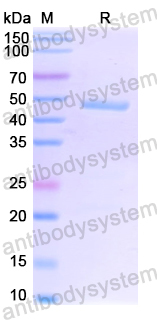
Purity
>90% as determined by SDS-PAGE.
Accession
UFO69287.1
Applications
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Form
Lyophilized
Storage buffer
Lyophilized from a solution in PBS pH 7.4, 0.02% NLS, 1mM EDTA, 4% Trehalose, 1% Mannitol.
Reconstitution
Reconstitute in sterile water for a stock solution. A copy of datasheet will be provided with the products, please refer to it for details.
Shipping
In general, proteins are provided as lyophilized powder/frozen liquid. They are shipped out with dry ice/blue ice unless customers require otherwise.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8°C for frequent use. Store at -20 to -80°C for twelve months from the date of receipt.
Alternative Names
Nucleoprotein, N, Nucleocapsid protein, NC, Protein N, N Protein, Nucleoprotein (Omicron), SARS-like Virus
Phylogeny-driven design of broadly protective sarbecovirus receptor-binding domain nanoparticle vaccines., PMID:40463102
A Nanomaterial-Independent Biosensor Based on Gallium Arsenide High-Electron-Mobility Transistors for Rapid and Ultra-Sensitive Pathogen Detection., PMID:40461437
Intrinsic factors behind long COVID: exploring the role of nucleocapsid protein in thrombosis., PMID:40416618
Natural product sennoside B disrupts liquid-liquid phase separation of SARS-CoV-2 nucleocapsid protein by inhibiting its RNA-binding activity., PMID:40371698
Transfer of SARS-CoV-2 nucleocapsid protein to uninfected epithelial cells induces antibody-mediated complement deposition., PMID:40343796
Self-assembled epitope-based nanoparticles targeting the SARS-CoV-2 spike protein enhanced the immune response and induced potential broad neutralizing activity., PMID:40270771
Antimicrobial protection of fabrics with poly(allylamine hydrochloride)-ZnO coating., PMID:40226926
Optimization of A549 Cell Transfection Efficiency with a Plasmid Encoding the N-Protein of the SARS-CoV-2 Virus., PMID:40216710
Targeting USP22 to promote K63-linked ubiquitination and degradation of SARS-CoV-2 nucleocapsid protein., PMID:40183543
Head-to-head comparison of anterior nares and nasopharyngeal swabs for SARS-CoV-2 antigen detection in a community drive-through test centre in the UK., PMID:40122534
Modulation of lipid nanoparticle-formulated plasmid DNA drives innate immune activation promoting adaptive immunity., PMID:40120578
Efficient one-step immobilization of DNA probes on 1DZnO nanoplatforms targeting a low-mutation region of SARS-CoV-2., PMID:40096751
Predictive Insights Into Bioactive Compounds from Streptomyces as Inhibitors of SARS-CoV-2 Mutant Strains by Receptor Binding Domain: Molecular Docking and Dynamics Simulation Approaches., PMID:40066112
Remdesivir and Obeldesivir Retain Potent Antiviral Activity Against SARS-CoV-2 Omicron Variants., PMID:40006923
Immunoassay Detection of SARS-CoV-2 Using Monoclonal Antibody Binding to Viral Nucleocapsid Protein., PMID:39989430
Concordance of maternal and cord blood SARS-COV-2 immunoglobulin seropositivity after COVID-19 infection or vaccination in pregnancy., PMID:39973524
Pre-vaccination immune markers predict response to BNT162b2 mRNA vaccine in vulnerable groups - The CONVERS project, report from a pediatric tertiary hospital., PMID:39947073
Co-administration of seasonal quadrivalent influenza and COVID-19 vaccines leads to enhanced immune responses to influenza virus and reduced immune responses to SARS-CoV-2 in naive mice., PMID:39921982
Immunogenicity and safety study of a single dose of SpikoGen® vaccine as a heterologous or homologous intramuscular booster following a primary course of mRNA, adenoviral vector or recombinant protein COVID-19 vaccine in ambulatory adults., PMID:39914274
Caspase-1 activation, IL-1/IL-6 signature and IFNγ-induced chemokines in lungs of COVID-19 patients., PMID:39882243
Molecular mechanisms behind the anti corona virus activity of small metal oxide nanoparticles., PMID:39846258
The SARS-CoV-2 nucleocapsid protein interferes with the full enzymatic activation of UPF1 and its interaction with UPF2., PMID:39831305
Spatiotemporal Dynamic Immunomodulation by Infection-Mimicking Gels Enhances Broad and Durable Protective Immunity Against Heterologous Viruses., PMID:39804984
A comparison between different chemical fractionation methods for immunoglobulin preparation., PMID:39799401
SARS-CoV-2 Infection of the Central Nervous System: A Case Report., PMID:39772268
A Global Collaborative Comparison of SARS-CoV-2 Antigenicity Across 15 Laboratories., PMID:39772242
Fast and Sensitive Detection of Anti-SARS-CoV-2 IgG Using SiO2@Au@CDs Nanoparticle-Based Lateral Flow Immunoassay Strip Coupled with Miniaturized Fluorimeter., PMID:39766275
Lab-on-the-Needles: A Microneedle Patch-Based Mobile Unit for Highly Sensitive Ex Vivo and In Vivo Detection of Protein Biomarkers., PMID:39763125
Non-spike protein inhibition of SARS-CoV-2 by natural products through the key mediator protein ORF8., PMID:39744515
Structural serology of polyclonal antibody responses to mRNA-1273 and NVX-CoV2373 COVID-19 vaccines., PMID:39713412
SARS-CoV-2 resistance analyses from the Phase 3 PINETREE study of remdesivir treatment in nonhospitalized participants., PMID:39699245
PET Foils Functionalized with Reactive Copolymers as Adaptable Microvolume ELISA Spot Array Platforms for Multiplex Serological Analysis of SARS-CoV-2 Infections., PMID:39686303
Magnetophoretic slider assay for electrochemical detection of SARS-cov-2 nucleocapsid protein in nasal swab samples., PMID:39671962
Protein nanoparticle vaccines induce potent neutralizing antibody responses against MERS-CoV., PMID:39644492
Immunoreactivity Analysis of MHC-I Epitopes Derived from the Nucleocapsid Protein of SARS-CoV-2 via Computation and Vaccination., PMID:39591116
Staggered immunization with mRNA vaccines encoding SARS-CoV-2 polymerase or spike antigens broadens the T cell epitope repertoire., PMID:39589869
Occult COVID-19 in an immunosuppressed patient with migratory pulmonary infiltrates., PMID:39571785
Dexamethasone-loaded chitosan-decorated PLGA nanoparticles: A step forward in attenuating the COVID-19 cytokine storm?, PMID:39522287
Au@Pt@Pd nanozymes based lateral flow immunoassay for quantitative detection of SARS-CoV-2 nucleocapsid protein in nasal swab samples., PMID:39508966
Effect of carbon black and silicon dioxide nanoparticle exposure on corona receptor ACE2 and TMPRSS2 expression in the ocular surface., PMID:39506016
Monitoring humoral responses against three SARS-CoV-2 vaccines in a university population from Chihuahua, Mexico., PMID:39499757
T Cell Peptide Prediction, Immune Response, and Host-Pathogen Relationship in Vaccinated and Recovered from Mild COVID-19 Subjects., PMID:39456150
Correlation of patient symptoms with SARS-CoV-2 Omicron variant viral loads in nasopharyngeal and saliva samples and their influence on the performance of rapid antigen testing., PMID:39382283
Potent neutralization of SARS-CoV-2 variants by RBD nanoparticle and prefusion-stabilized spike immunogens., PMID:39379400
Enhancing Omicron Sublineage Neutralization: Insights From Bivalent and Monovalent COVID-19 Booster Vaccines and Recent SARS-CoV-2 Omicron Variant Infections., PMID:39377176
Effects of age and gender on immunogenicity and reactogenicity of SpikoGen recombinant spike protein vaccine: a post-hoc analysis., PMID:39349494
Differential detection of SARS-CoV-2 variants and influenza A viruses utilizing a dual lateral flow strip based on colloidal gold-labeled monoclonal antibodies., PMID:39341304
Oral SARS-CoV-2 host responses predict the early COVID-19 disease course., PMID:39294156
Post-Hoc Analysis of Potential Correlates of Protection of a Recombinant SARS-CoV-2 Spike Protein Extracellular Domain Vaccine Formulated with Advax-CpG55.2-Adjuvant., PMID:39273405
Viral, Immunologic, and Laboratory Parameters in Patients With and Without Post-Acute Sequelae of SARS-CoV-2 Infection (PASC)., PMID:39252682