Catalog No.
KDV02803
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
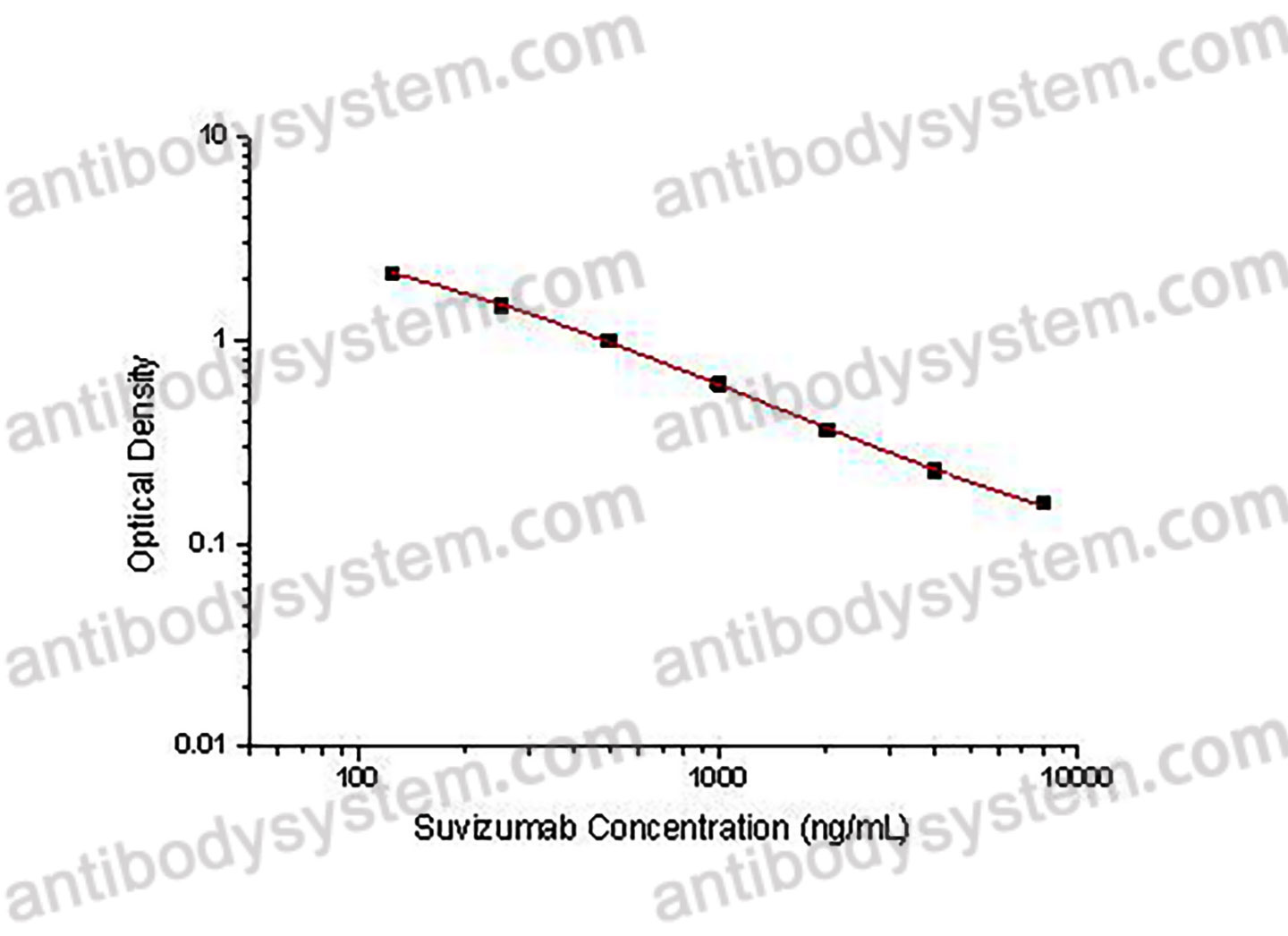
0.31-5 μg/mL
Sensitivity
0.156 μg/ml
Precision
CV<20%
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
The stability of ELISA kit is determined by the loss rate of activity. The loss rate of this kit is less than 10% prior to the expiration date under appropriate storage condition.
Alternative Names
H1H3592P3, REGN 2222, SAR-438584, SAR438584,REGN2222,CAS: 1629615-23-1
Human iPS cell-derived respiratory organoids as a model for respiratory syncytial virus infection., PMID:40262853
Amino acid substitutions in the fusion protein of respiratory syncytial virus in Fukushima, Japan during 2008-2023 and their effects., PMID:39719011
Systematic Review of the Efficacy and Safety of RSV-Specific Monoclonal Antibodies and Antivirals in Development., PMID:39209729
Genotype Analysis of Respiratory Syncytial Virus Before and After the COVID-19 Pandemic Using Whole-Genome Sequencing: A Prospective, Single-Center Study in Korea From 2019 to 2022., PMID:39048301
Genetic characterization of respiratory syncytial virus surface glycoproteins F and G in Taiwan, 2017-2021., PMID:38937186
Monoclonal Antibody for the Prevention of Respiratory Syncytial Virus in Infants and Children: A Systematic Review and Network Meta-analysis., PMID:36800182
A systematic review on global RSV genetic data: Identification of knowledge gaps., PMID:34543489
Suptavumab for the Prevention of Medically Attended Respiratory Syncytial Virus Infection in Preterm Infants., PMID:32897368
Respiratory Syncytial Virus: Targeting the G Protein Provides a New Approach for an Old Problem., PMID:29118126
Antibodies to watch in 2017., PMID:27960628