Catalog No.
KDA30301
Description
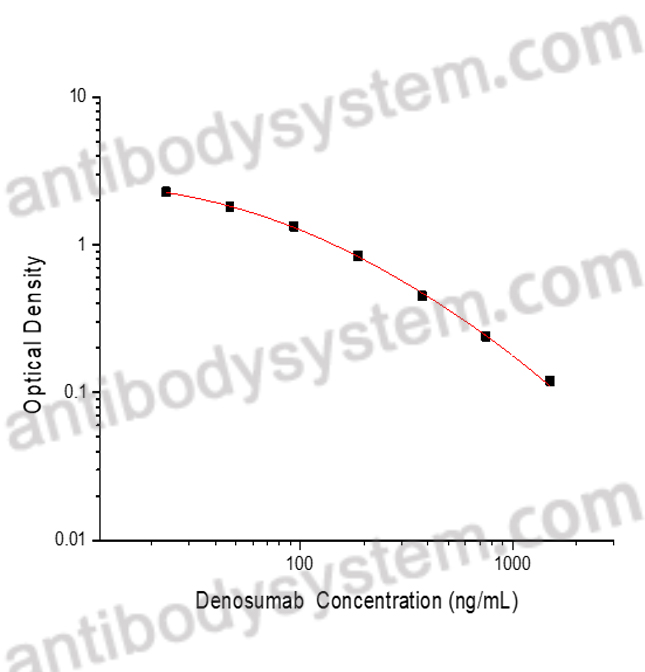
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD254 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Denosumab in the sample competitively binds to the pre-coated protein with biotin-labeled Denosumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Denosumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Denosumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
23.44 - 1,500 ng/mL
Sensitivity
12.21 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
858.2
|
164.6
|
31.9
|
817.5
|
149.0
|
36.4
|
|
Standard deviation
|
103.2
|
29.9
|
4.9
|
76.8
|
22.8
|
5.2
|
|
CV (%)
|
12.0
|
18.2
|
15.3
|
9.4
|
15.3
|
14.3
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
AMG162, HL2001,CAS: 615258-40-7
Osteoporosis Management after the Occurrence of Medication-Related Osteonecrosis of the Jaw: A 13-Year Experience at a Tertiary Center., PMID:40509705
Denosumab-bbdz: A Review of the Interchangeable Biosimilar for the Treatment of Osteoporosis., PMID:40509651
RANKL Drives Bone Metastasis in Mammary Cancer: Protective Effects of Anti-Resorptive Treatments., PMID:40507804
Bisphosphonate beak without the bisphosphonate: atypical femoral fracture with denosumab., PMID:40506101
Dental implant failure and medication-related osteonecrosis of the jaw (MRONJ) related to dental implants in patients taking antiresorptive therapy for osteoporosis: a systematic review and meta-analysis., PMID:40505730
Patterns of adjuvant bone modifying agent use in patients with early-stage breast cancer in the United States., PMID:40500761
Prolonged hypercalcemia induced by teriparatide: a case report and literature review., PMID:40500389
Delayed-onset hypocalcaemia and hypophosphataemia induced by iron infusion in the context of denosumab therapy., PMID:40499946
Severe immunotherapy-related thrombocytopenia in metastatic bone cancer: a multicenter retrospective case series highlighting early recognition and management., PMID:40496609
Denosumab in patients with osteogenesis imperfecta and a historical control study with alendronate., PMID:40496553
The prevention of osteoporotic vertebral fractures in eastern and in western countries., PMID:40495909
Fragility of Evidence for the Efficacy of Anti-fracture Medications., PMID:40488289
Moderate-to-severe hypercalcemia secondary to primary hyperparathyroidism refractory to conventional treatment (therapeutic management with denosumab): case report and literature review., PMID:40486005
30 YEARS OF BONE GIANT CELL TUMOR IN THE KNEE: A BRAZILIAN PERSPECTIVE., PMID:40475359
Comparative efficacy of teriparatide and bisphosphonates or denosumab vs. teriparatide monotherapy in osteoporosis: a meta-analysis., PMID:40469971
Diagnosis of skeletal fragility due to Loeys-Dietz syndrome and treatment with romosozumab followed by denosumab., PMID:40469084
Primary Hyperparathyroidism with Severe Vitamin D Deficiency in Chronic Kidney Disease., PMID:40467506
Asia-Pacific consensus for the management of osteoporosis in men., PMID:40464984
Novel approach to central giant cell granuloma of the Jaw: Low-dose intralesional denosumab treatment in a case series of nine patients., PMID:40461338
Impact of denosumab on muscle health in older adults in long-term care., PMID:40460961
Enzalutamide plus radium-223 in metastatic castration-resistant prostate cancer: results of the EORTC 1333/PEACE-3 trial., PMID:40450503
Atypical femoral fracture associated with sequential zoledronic acid and denosumab therapy: a case report., PMID:40459674
A missense mutation in close proximity of ALS-linked PFN1 mutations causes only early-onset Paget's disease of bone., PMID:40458045
Treatment of Osteoporosis in Patients with Chronic Kidney Disease., PMID:40457078
The link between osteoporosis and cardiovascular diseases: a review of shared mechanisms, risk factors, and therapeutic approaches., PMID:40455217
Long-term effectiveness and safety of denosumab for osteoporosis in patients with rheumatic diseases., PMID:40451270
Use of Low-Value Cancer Treatments in Medicare Advantage Versus Traditional Medicare., PMID:40448575
Characteristics and treatment options of 272,152 geriatric patients with very high and high fracture risk., PMID:40445411
Comparison of Type 2 Diabetes Risk in Osteoporosis Patients Treated with Denosumab or Alendronate: A Nationwide Cohort Study., PMID:40445395
EXPRESS: Osteoblasts inhibit NK cell killing function via RANK/RANKL in multiple myeloma., PMID:40444878
A practical use of bone turnover markers in management of patients with skeletal fragility., PMID:40439877
Understanding and Managing Fracture Risk in Patients With Cancer: A Literature Review., PMID:40438830
Five-Year Sales Trends of Osteoporosis Medications in Korea: A Market Analysis Based on IMS Health Sales Audit Data (2018-2023)., PMID:40428763
Supra-Physiological Levels of Magnesium Counteract the Inhibitory Effect of Zoledronate on RANKL-Dependent Osteoclastogenesis., PMID:40427722
Comprehensive Evaluation of Treatment Patterns in Postmenopausal Patients with Osteoporosis without Fractures: Insights from Tertiary Care Institutions and Nationwide OMOP-CDM Data., PMID:40426310
Mouse model of anti-RANKL discontinuation reveals reduced bone mass and quality through disruption of bone remodeling., PMID:40425548
Comparative analysis of Denosumab and Zoledronic acid in advanced breast cancer patients receiving CDK4/6 inhibitors., PMID:40424680
Denosumab and Fracture Prevention in Primary Care Practice., PMID:40423959
Symptomatic hypocalcaemia after administration of denosumab and iron infusion in patients with normal and impaired renal function., PMID:40423532
Role of denosumab in lipid metabolism disorders: clinical significance and potential mechanisms., PMID:40418391
No overshoot of bone turnover after withdrawal of denosumab treatment of adults with Langerhans cell histiocytosis: a prospective clinical trial., PMID:40418338
Evaluation of Anti-SARS-CoV-2 IgG Responses in a Clinical Study of a Biosimilar Candidate to Denosumab Using Singlicate Analysis., PMID:40408051
Effect of denosumab on semen quality in infertile men selected by serum level of antimüllerian hormone: a randomized controlled trial., PMID:40403914
Denosumab treatment of giant cell tumors in the spine induces woven bone formation., PMID:40390810
Real-world differences in denosumab persistence, reinitiation, and switching among cohorts of older adults in Canada and the United States., PMID:40390804
Incidence of medication-related osteonecrosis of the jaw and associated antiresorptive drugs in adult Finnish population., PMID:40389539
A comparative analysis of Denosumab and Zoledronic acid effects on bone metabolism and bone mineral density in individuals with osteoporotic vertebral compression fractures., PMID:40386518
Spinal giant cell tumour presenting as a posterior mediastinal mass., PMID:40379300
Agents to treat osteoporosis in chronic kidney disease., PMID:40377654
Monitoring denosumab therapy using the calcium isotope marker (CIM) technology., PMID:40374024