Catalog No.
KDD72201
Description
PRINCIPLE OF THE ASSAY This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD27 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Varlilumab in the sample competitively binds to the pre-coated protein with biotin-labeled Varlilumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Varlilumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
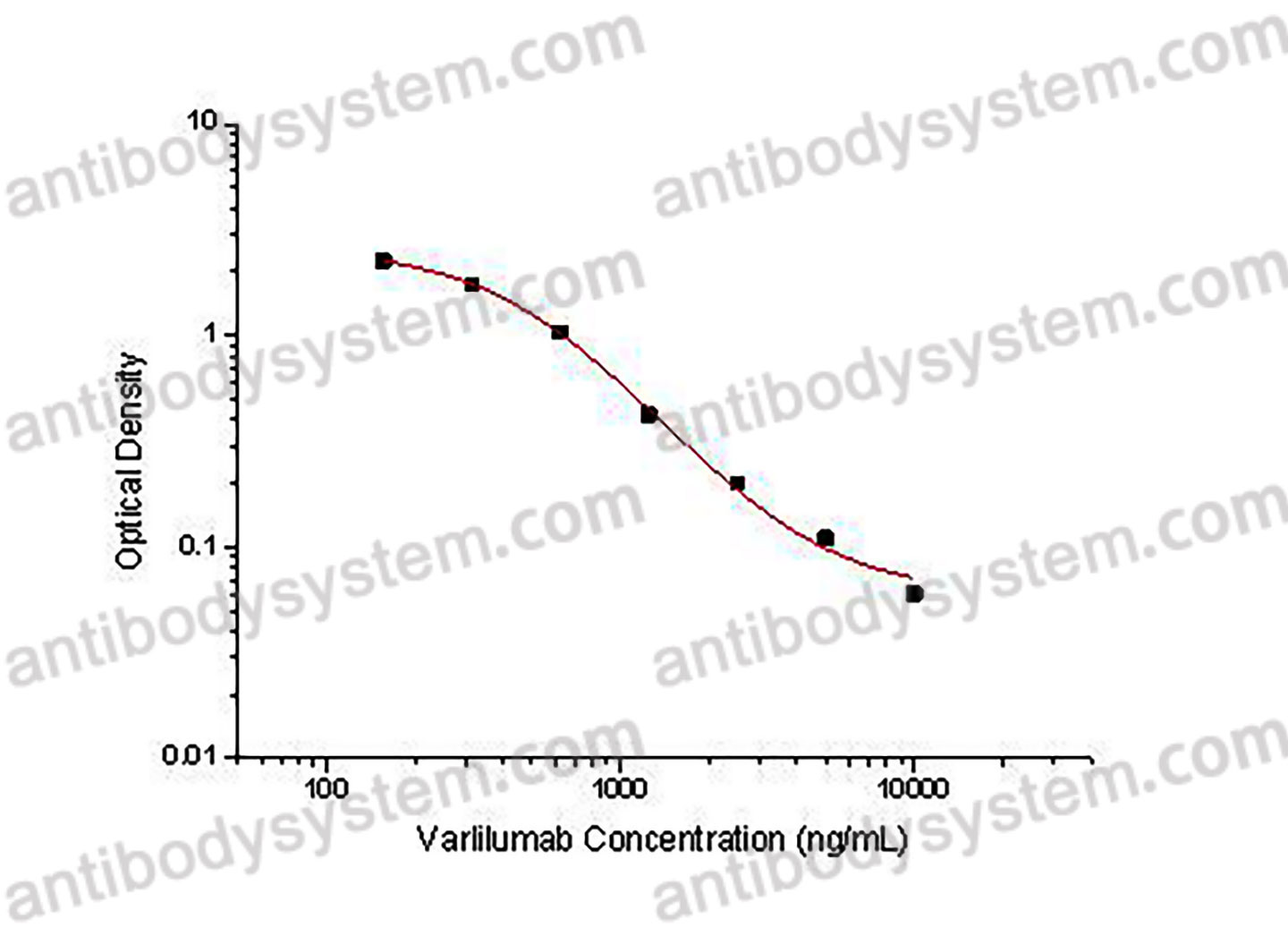
Used for the quantitative determination of Varlilumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
156.25 - 10,000 ng/mL
Sensitivity
173.67 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision |
Inter-Assay Precision |
||||
|
Sample |
1 |
2 |
3 |
1 |
2 |
3 |
|
n |
16 |
16 |
16 |
24 |
24 |
24 |
|
Mean (ng/mL) |
5155.4 |
1253.4 |
453.7 |
5100.9 |
1304.5 |
526.2 |
|
Standard deviation |
408.6 |
81.5 |
32.5 |
443.0 |
89.2 |
59.1 |
|
CV (%) |
7.9 |
6.5 |
7.2 |
8.7 |
6.8 |
11.2 |
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
1F5, CDX-1127, CAS: 1393344-72-3
Background
Varlilumab (CDX-1127), a human IgG1κ monoclonal antibody, targets CD27 receptor through reacting with the ligand binding site of CD27. CD27 is a member of the tumor necrosis factor receptor superfamily and expressed on unstimulated T lymphocytes, serving as a potent costimulatory molecule. It has been found that CD27 induces a series of T-cell reactions including T-cell activation, proliferation, survival, and maturation of effector capacity and T-cell memory together with its ligand CD70, which is transiently expressed on antigen-presenting cells. Besides, the interaction of CD27 and CD70 also stimulate B-cell proliferation, generation of plasma cells, production of immunoglobulin and B-cell memory, and induction of the cytolytic activity of natural killer (NK) cells. Moreover, CD27 is considered to function on tumor expansion and activation as it also expressed by regulatory T cells (Tregs), a cell that is associated with the suppression of antitumor immunity. The drug, developed by Celldex Therapeutics, is designed for immunotherapy for patients with solid tumors and hematologic malignancies. Varlilumab is currently conducted with two trails. One is the phase 1B clinical trial for advanced breast or ovarian cancer in combination with ONT-10. Another is the phase 1/2 trial for advanced refractory solid tumors together with anti-PD-1 nivolumab.