Catalog No.
KDD80801
Description
PRINCIPLE OF THE ASSAY
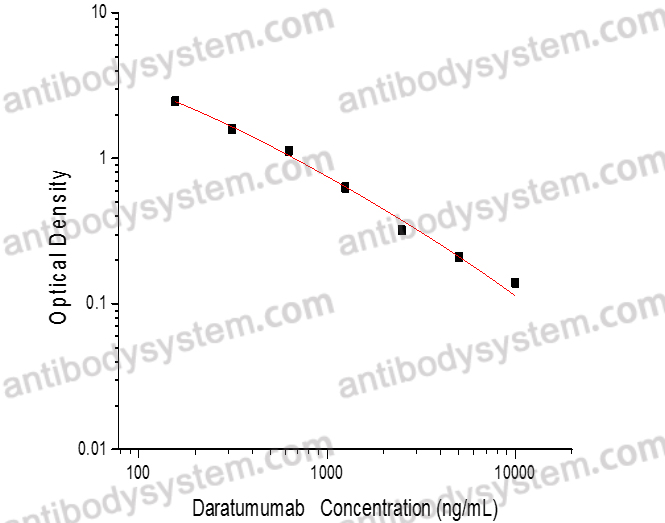
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD38 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Daratumumab in the sample competitively binds to the pre-coated protein with biotin-labeled Daratumumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Daratumumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Daratumumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
156.25 - 10,000 ng/mL
Sensitivity
104.76 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
5609.9
|
1212.3
|
403.2
|
5084.5
|
1092.0
|
402.8
|
|
Standard deviation
|
386.9
|
120.9
|
35.1
|
630.7
|
109.6
|
59.7
|
|
CV (%)
|
6.9
|
10.0
|
8.7
|
12.4
|
10.0
|
14.8
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20 ℃, the rest reagents should be store at 4℃.
Alternative Names
HuMax-CD38, CAS: 945721-28-8
Background
Daratumumab is human IgG1 monoclonal antibody to CD38, which is a transmembrane glycoprotein that is frequently overexpressed on cancer cells including multiple myeloma cells. The monoclonal antibody binds to the CD38 molecule and triggers cell apoptosis, probably as a result of antibody mediated cytotoxicity. Daratumumab has been evaluated in heavily pretreated patients with refractory multiple myeloma and shown overall response rates of higher than expected. Daratumumab was given accelerated approval in the United States in 2015 for use in multiple myeloma. Current indications are as therapy of patients with refractory multiple myeloma in combination with lenalidomide (or bortezomib) and dexamethasone or as monotherapy in patients who have failed at least three previous regimens. Daratumumab is available as a solution for intravenous infusion in single use vials of 100 mg in 5 mL or 400 mg in 20 mL (20 mg/mL). The recommended dose is 16 mg/kg intravenously every week for 8 to 9 weeks, and then every 2, 3 or 4 weeks based upon indications and other agents being used. Premedication with methylprednisolone is recommended. Side effects are common and can include infusion reactions, bone marrow suppression, fatigue, nausea and vomiting, diarrhea, muscle spasms, back pain, fever, cough, dyspnea, peripheral edema, peripheral neuropathy and upper respiratory infection. Rare, but potentially serious side effects include severe infusion reactions, neutropenia, thrombocytopenia and interference with cross matching and red blood cell antibody screening.
Quantification of serum daratumumab in multiple myeloma patients by LC-MS/MS, comparison with ELISA. [KDD80801]
Association of plasma n-3 polyunsaturated fatty acid with gut mycobiome and implications for glucose homeostasis., PMID:40516019
In-situ Visualization of Level 3 LFPs Using a Lipophilic Red AIE Fluorescent Probe with a D-A Structure., PMID:40516016
Effect of hydrochemical conditions on transport of HFPO-DA in saturated quartz sand and limestone porous media., PMID:40515870
Context-dependent body size evolution in lacertid lizards: differential role of structural habitat and climate across radiations., PMID:40515787
'I'm Not a Bad Mom, I'm a Sick Mom': Using Photovoice to Examine Parental Recovery Narratives Given Institutional Constraints., PMID:40515630
Predicting Treatment Outcome in Congenital Adrenal Hyperplasia Using Urine Steroidomics and Machine Learning., PMID:40515610
Addressing the mean-variance relationship in spatially resolved transcriptomics data with spoon., PMID:40515599
Sibling-Focused Family Prevention With Latinx Siblings in Middle Childhood: A Randomized Clinical Trial Spanning the COVID-19 Pandemic., PMID:40515523
How Do Dental Professionals and Students Assess Orthodontic Case Complexity?, PMID:40515439
KansformerEPI: a deep learning framework integrating KAN and transformer for predicting enhancer-promoter interactions., PMID:40515390
Highly Selective Electrochemical Detection of Vitamin K1 (Phylloquinone) in Simulated Blood Serum Using Bimetallic Cu/Ni-MOF Decorated CNT Composite on Nickel Foam., PMID:40514770
Multi-trait/environment sparse genomic prediction using the SFSI R-package., PMID:40514769
Composition and rhythmic variations in the microbiome of Southwestern Atlantic corals., PMID:40514749
Secondary analysis of the EMPACT-MI trial reveals cardiovascular-kidney efficacy and safety of empagliflozin after acute myocardial infarction., PMID:40514435
Directing negative emotional states through parallel genetically-distinct basolateral amygdala pathways to ventral striatum subregions., PMID:40514388
Design, construction and characterization of laccase-xylanase chimeras by insertional fusion., PMID:40514177
Predictive value of preclinical models for CAR-T cell therapy clinical trials: a systematic review and meta-analysis., PMID:40514065
Experiences of Gender and Racial/Ethnic Discrimination in Sexual Healthcare Among Transgender and Nonbinary Young Adults of Color., PMID:40514048
Endoscopic submucosal dissection of early gastric cancer in the gastric fundus: challenges and techniques., PMID:40514029
In silico and in vitro screening of medicinal plants from Brazilian traditional medicine for anti-Helicobacter pylori activity., PMID:40514023
Association Between Preoperative Anemia and the Risk of Revision Following Total Knee Arthroplasty: A Multi-Institutional Retrospective Study., PMID:40513902
Local recurrence rates of horizontal margin-positive en bloc endoscopic submucosal dissection of colorectal neoplasia: a meta-analysis., PMID:40513803
Natural polysaccharide mediated hybrid intumescent flame retardant enables epoxy resin with high fire safety and mechanical performance., PMID:40513750
Exploring the protective mechanism of Lentinus edodes mycelium polysaccharide against AGEs-induced HUVECs damage: Insights from whole transcriptome sequencing and cell biology techniques., PMID:40513741
Oral delivery of tunable oxidation-responsive budesonide-loaded nanoparticles enhances inflammation modulation in intestinal colitis., PMID:40513667
Exposure to cadmium alters metabolic pathways in the hepatic proteome of a neotropical catfish., PMID:40513393
Nivolumab plus relatlimab in advanced melanoma: RELATIVITY-047 4-year update., PMID:40513285
Influence of Soil Characteristics on the Phytochemistry of Evergreen Ivy (Hedera helix L.) Leaves in Deciduous Forests., PMID:40513112
Investigating Cavity Quantum Electrodynamics-Enabled Endo/Exo-Selectivities in a Diels-Alder Reaction., PMID:40513107
Aberrant Right Vertebral Artery with Kommerell's Diverticulum: An Unique Lusoria Variant., PMID:40513104
Parasacral Transcutaneous Electrical Nerve Stimulation with Desmopressin Acetate for Treating Primary Monosymptomatic Enuresis: A Randomized Controlled Clinical Trial., PMID:40513022
Mycorrhizal symbioses and tree diversity in global forest communities., PMID:40512852
Eco-friendly degreasing adsorbent derived from oily scum and walnut shells for oilfield sewage treatment and industrial oils adsorption., PMID:40512703
Impact of Molecular Crowding on Accessibility of Telomeric Overhangs Forming Multiple G-Quadruplexes., PMID:40512671
Effects of concurrent training on maximal and explosive strength in trained individuals: Insights from the load-velocity relationship., PMID:40512632
Enhancing Monte Carlo Tree Search for Retrosynthesis., PMID:40512567
MicroMundo@Oeiras: Citizen Science promoting antibiotic stewardship, discovery of new antimicrobials and monitoring of soil resistance., PMID:40512524
The Impact of Yoga Practice on Health, Strength, and Respiratory Capacity in Portuguese Airforce Pilots: an Applied Psychophysiology and Biofeedback Approach., PMID:40512335
Optimized prism-based surface plasmon resonance sensor for malaria detection: role of 2D nanomaterials for an enhancement in sensitivity and accuracy., PMID:40512247
Protective Effect of Selegiline (R-deprenyl) in Aminoglycoside-Induced Hearing Loss., PMID:40512245
A multivariate analysis examining the relationship between sociodemographic differences and UK graduates' performance on postgraduate medical exams., PMID:40512226
[Theory-driven examination of available data on age(ing) and climate change: an explorative contribution]., PMID:40512225
From survival of irradiated mice to modern molecular insights: a seventy-year journey in radiobiology at the institute of biophysics, Czech academy of sciences., PMID:40512188
The challenging approach to the management of male partners of HPV-positive women., PMID:40511955
Comparing subjective effects and intoxication on simultaneous alcohol and cannabis use occasions relative to alcohol- or cannabis-only occasions., PMID:40511871
A Synthetic Route to Functionalized Thiopyran Derivatives via Domino Ring-Opening Cyclization (DROC) of Donor-Acceptor Cyclopropanes with Pyridinium Thiolates., PMID:40511864
Therapeutic Potential of PSC-derived Cell Transplantation in Parkinson's Disease: A Systematic Review and Meta-analysis of Preclinical Studies., PMID:40511801
Synthesis and Evaluation of Mesoporous Silica-Biopolymer-Based Bone Substitutes for Tissue Engineering., PMID:40511501
The Centriole Stability Assay: A Method to Investigate Mechanisms Involved in the Maintenance of the Centrosome Structure in Drosophila Cultured Cells., PMID:40511405
Characterization of Xiaoqu Qingxiangxing Baijiu by gas chromatography-olfactometry, quantitative measurements, aroma recombination, and omission experiments., PMID:40511332