Catalog No.
KDC09604
Description
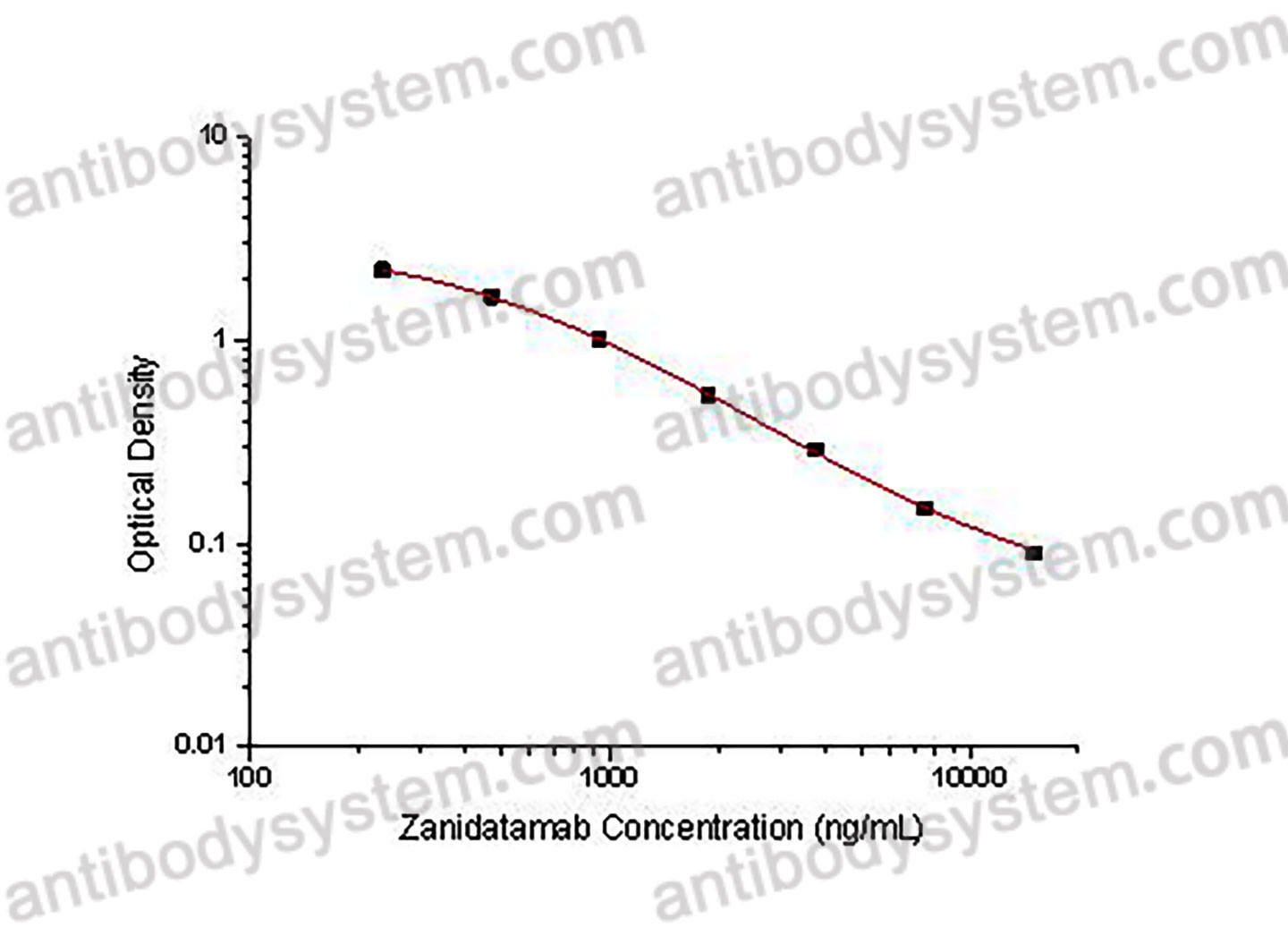
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD340 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Zanidatamab in the sample competitively binds to the pre-coated protein with biotin-labeled Zanidatamab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Zanidatamab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Zanidatamab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
234.38 - 15,000 ng/mL
Sensitivity
120.41 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
7471.0
|
1734.1
|
516.4
|
7795.0
|
1755.1
|
401.5
|
|
Standard deviation
|
1020.1
|
88.0
|
42.9
|
1113.9
|
103.8
|
49.8
|
|
CV (%)
|
13.7
|
5.1
|
8.3
|
14.3
|
5.9
|
12.4
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
ZW-25, ZW25, zanidatamab zovodotin, CAS: 2169946-15-8
Background
zanidatamab is a novel her2-targeted antibody that binds two distinct extracellular domains of her2, allowing for multiple mechanisms of action including enhanced binding, clustering, receptor internalization and downregulation
Zanidatamab, a bispecific monoclonal antibody for HER2-positive gastro-oesophageal adenocarcinoma patients: a new hope?, PMID:40473446
Zanidatamab plus chemotherapy as first-line treatment for patients with HER2-positive advanced gastro-oesophageal adenocarcinoma: primary results of a multicentre, single-arm, phase 2 study., PMID:40473445
Zanidatamab monotherapy or combined with chemotherapy in HER2-expressing gastroesophageal adenocarcinoma: a phase 1 trial., PMID:40341383
Zanidatamab plus palbociclib and fulvestrant in previously treated patients with hormone receptor-positive, HER2-positive metastatic breast cancer: primary results from a two-part, multicentre, single-arm, phase 2a study., PMID:40339592
Real-world efficacy of zanidatamab in patients with HER2 positive advanced biliary tract cancers., PMID:40319675
Safety and Efficacy of Anti-Human Epidermal Growth Factor 2 Agents in the Treatment of Biliary Tract Cancers: A Systematic Review., PMID:40239136
Zanidatamab: First Approval., PMID:40108069
Exploring Zanidatamab's efficacy across HER2-positive Malignancies: a narrative review., PMID:40025472
Novel drugs approved by the EMA, the FDA and the MHRA in 2024: A year in review., PMID:39971274
Zanidatamab (Ziihera) for biliary tract cancer., PMID:39819991
Antibodies to watch in 2025., PMID:39711140
[What's new in gastric cancer?]., PMID:39146748
Advances in HER2-Targeted Therapies: From monoclonal antibodies to dual inhibitors developments in cancer treatment., PMID:39137598
A plain language summary of the results from the phase 2b HERIZON-BTC-01 study of zanidatamab in participants with HER2-amplified biliary tract cancer., PMID:39114870
State of the art and upcoming trends in HER2-directed therapies in gastrointestinal malignancies., PMID:38726843
Co-clinical Trial of Novel Bispecific Anti-HER2 Antibody Zanidatamab in Patient-Derived Xenografts., PMID:38358339
Revolutionizing anti-HER2 therapies for extrahepatic cholangiocarcinoma and gallbladder cancer: Current advancements and future perspectives., PMID:38266541
A phase 2 trial of zanidatamab in HER2-overexpressed advanced endometrial carcinoma and carcinosarcoma (ZW25-IST-2)., PMID:38262242
New developments and standard of care in the management of advanced gastric cancer., PMID:37952913
Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study., PMID:37276871
An anti-HER2 biparatopic antibody that induces unique HER2 clustering and complement-dependent cytotoxicity., PMID:36914633
Antibodies to watch in 2023., PMID:36472472
Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose-escalation and expansion study., PMID:36400106
Population pharmacokinetics of zanidatamab, an anti-HER2 biparatopic antibody, in patients with advanced or metastatic cancer., PMID:36102999
HERIZON-GEA-01: Zanidatamab + chemo ± tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma., PMID:36000541
Current and emerging therapies for advanced biliary tract cancers., PMID:34626563