Catalog No.
KDC21002
Description
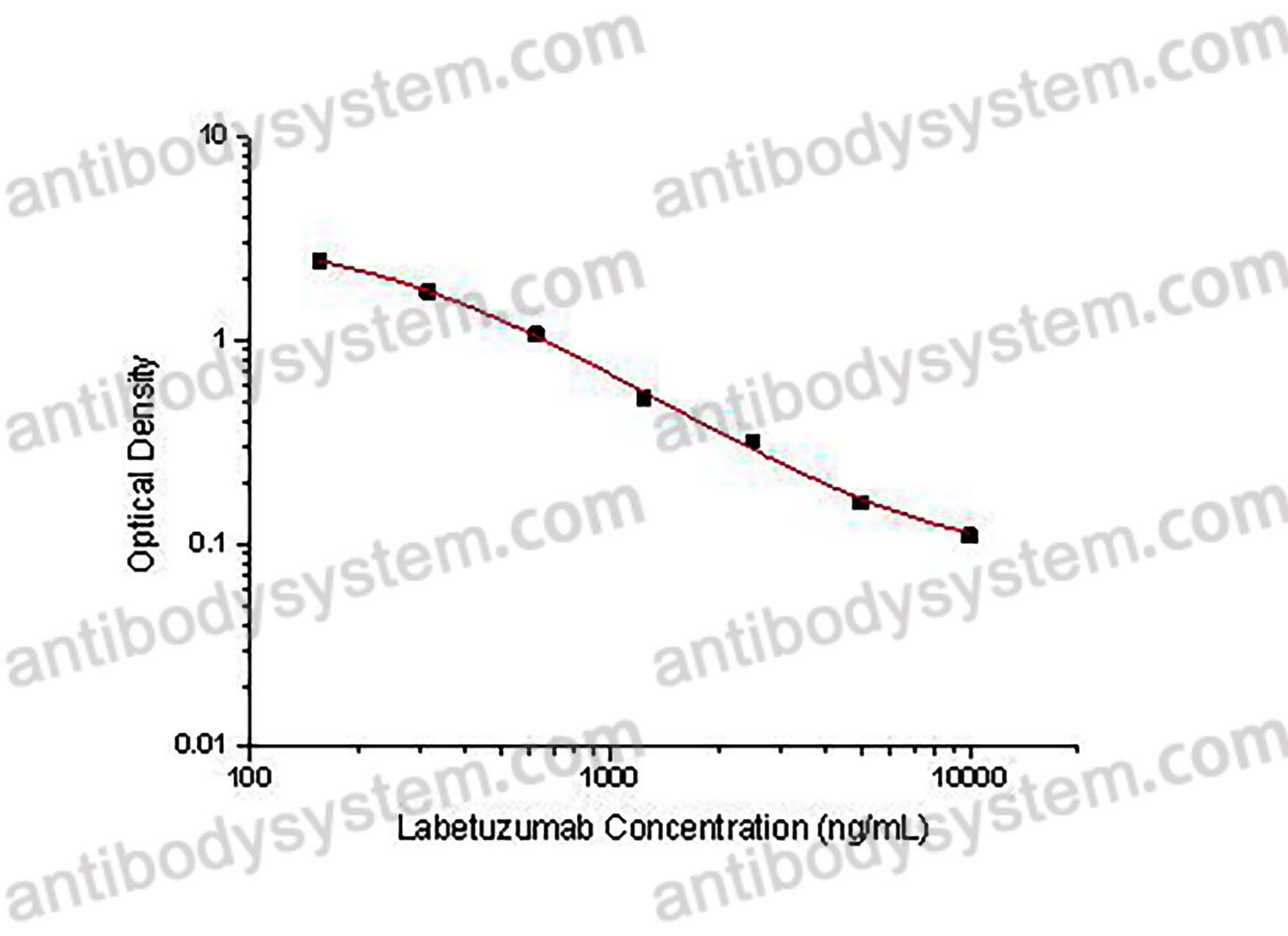
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD66e has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Labetuzumab in the sample competitively binds to the pre-coated protein with biotin-labeled Labetuzumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Labetuzumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Labetuzumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
156.25 - 10,000 ng/mL
Sensitivity
53.67 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
5125.6
|
1141.2
|
275.1
|
6291.1
|
1251.3
|
237.8
|
|
Standard deviation
|
527.8
|
55.2
|
28.7
|
705.2
|
159.7
|
19.8
|
|
CV (%)
|
10.3
|
4.8
|
10.4
|
11.2
|
12.8
|
8.3
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20 ℃, the rest reagents should be store at 4℃.
Alternative Names
hMN14, IMMU-130, hMN-14-SN-38, hMN-14-SN-38 ADC, hMN14-CL-SN-38, Labetuzumab Govitecan, CAS: 219649-07-7
Multimodal carcinoembryonic antigen-targeted fluorescence and radio-guided cytoreductive surgery for peritoneal metastases of colorectal origin: single-arm confirmatory trial., PMID:40270484
CEACAM5-Targeted Immuno-PET in Androgen Receptor-Negative Prostate Cancer., PMID:38782457
Multimodal CEA-targeted fluorescence and radioguided cytoreductive surgery for peritoneal metastases of colorectal origin., PMID:35551444
Regulation of CEACAM5 and Therapeutic Efficacy of an Anti-CEACAM5-SN38 Antibody-drug Conjugate in Neuroendocrine Prostate Cancer., PMID:33199493
Impact of Different Selectivity between Soluble and Membrane-bound Forms of Carcinoembryonic Antigen (CEA) on the Target-mediated Disposition of Anti-CEA Monoclonal Antibodies., PMID:31533926
Antibody-drug conjugates of 7-ethyl-10-hydroxycamptothecin: Sacituzumab govitecan and labetuzumab govitecan., PMID:30822636
Selective and Concentrated Accretion of SN-38 with a CEACAM5-Targeting Antibody-Drug Conjugate (ADC), Labetuzumab Govitecan (IMMU-130)., PMID:29079710
Labetuzumab govitecan in metastatic colorectal cancer., PMID:28844817
Phase I/II Trial of Labetuzumab Govitecan (Anti-CEACAM5/SN-38 Antibody-Drug Conjugate) in Patients With Refractory or Relapsing Metastatic Colorectal Cancer., PMID:28817371
Detection of Micrometastases Using SPECT/Fluorescence Dual-Modality Imaging in a CEA-Expressing Tumor Model., PMID:28126888
Repeated adjuvant anti-CEA radioimmunotherapy after resection of colorectal liver metastases: Safety, feasibility, and long-term efficacy results of a prospective phase 2 study., PMID:27763687
Improving the therapeutic index in cancer therapy by using antibody-drug conjugates designed with a moderately cytotoxic drug., PMID:25402018
SPECT- and fluorescence image-guided surgery using a dual-labeled carcinoembryonic antigen-targeting antibody., PMID:24982436
Multimodal treatment options for bilobar colorectal liver metastases., PMID:20213463
CEACAM5-targeted therapy of human colonic and pancreatic cancer xenografts with potent labetuzumab-SN-38 immunoconjugates., PMID:19789330
In vitro and in vivo anticancer efficacy of unconjugated humanized anti-CEA monoclonal antibodies., PMID:18728675
Radioimmunotherapy for liver metastases., PMID:17726786
Update of carcinoembryonic antigen radioimmunotherapy with (131)I-labetuzumab after salvage resection of colorectal liver metastases: comparison of outcome to a contemporaneous control group., PMID:17570017
Gateways to clinical trials., PMID:16636723
Phase II trial of carcinoembryonic antigen radioimmunotherapy with 131I-labetuzumab after salvage resection of colorectal metastases in the liver: five-year safety and efficacy results., PMID:16170184
Development of humanized antibodies as cancer therapeutics., PMID:15848077
A phase I trial combining high-dose 90Y-labeled humanized anti-CEA monoclonal antibody with doxorubicin and peripheral blood stem cell rescue in advanced medullary thyroid cancer., PMID:15809485
Gateways to clinical trials., PMID:15672123
A humanized monoclonal antibody to carcinoembryonic antigen, labetuzumab, inhibits tumor growth and sensitizes human medullary thyroid cancer xenografts to dacarbazine chemotherapy., PMID:15634649
Clinical-scale radiolabeling of a humanized anticarcinoembryonic antigen monoclonal antibody, hMN-14, with residualizing 131I for use in radioimmunotherapy., PMID:15632046
Carcinoembryonic antigen antibody inhibits lung metastasis and augments chemotherapy in a human colonic carcinoma xenograft., PMID:15592930
Carcinoembryonic antigen-immunoglobulin Fc fusion protein (CEA-Fc) for identification and activation of anti-CEA immunoglobulin-T-cell receptor-modified T cells, representative of a new class of Ig fusion proteins., PMID:15002034
Biotinylation, pharmacokinetics, and extracorporeal adsorption of humanized MAb 111In-MN14 using an avidin-affinity column in rats., PMID:12954123
Experimental radioimmunotherapy of small peritoneal metastases of colorectal origin., PMID:12918078
Phase I radioimmunotherapy trial with iodine-131--labeled humanized MN-14 anti-carcinoembryonic antigen monoclonal antibody in patients with metastatic gastrointestinal and colorectal cancer., PMID:12453334
Initial experience with high-dose radioimmunotherapy of metastatic medullary thyroid cancer using 131I-MN-14 F(ab)2 anti-carcinoembryonic antigen MAb and AHSCR., PMID:10647610
Phase I/II trial of (131)I-MN-14F(ab)2 anti-carcinoembryonic antigen monoclonal antibody in the treatment of patients with metastatic medullary thyroid carcinoma., PMID:10223579
Prospects of radioimmunotherapy in epithelial ovarian cancer: results with iodine-131-labeled murine and humanized MN-14 anti-carcinoembryonic antigen monoclonal antibodies., PMID:9441773