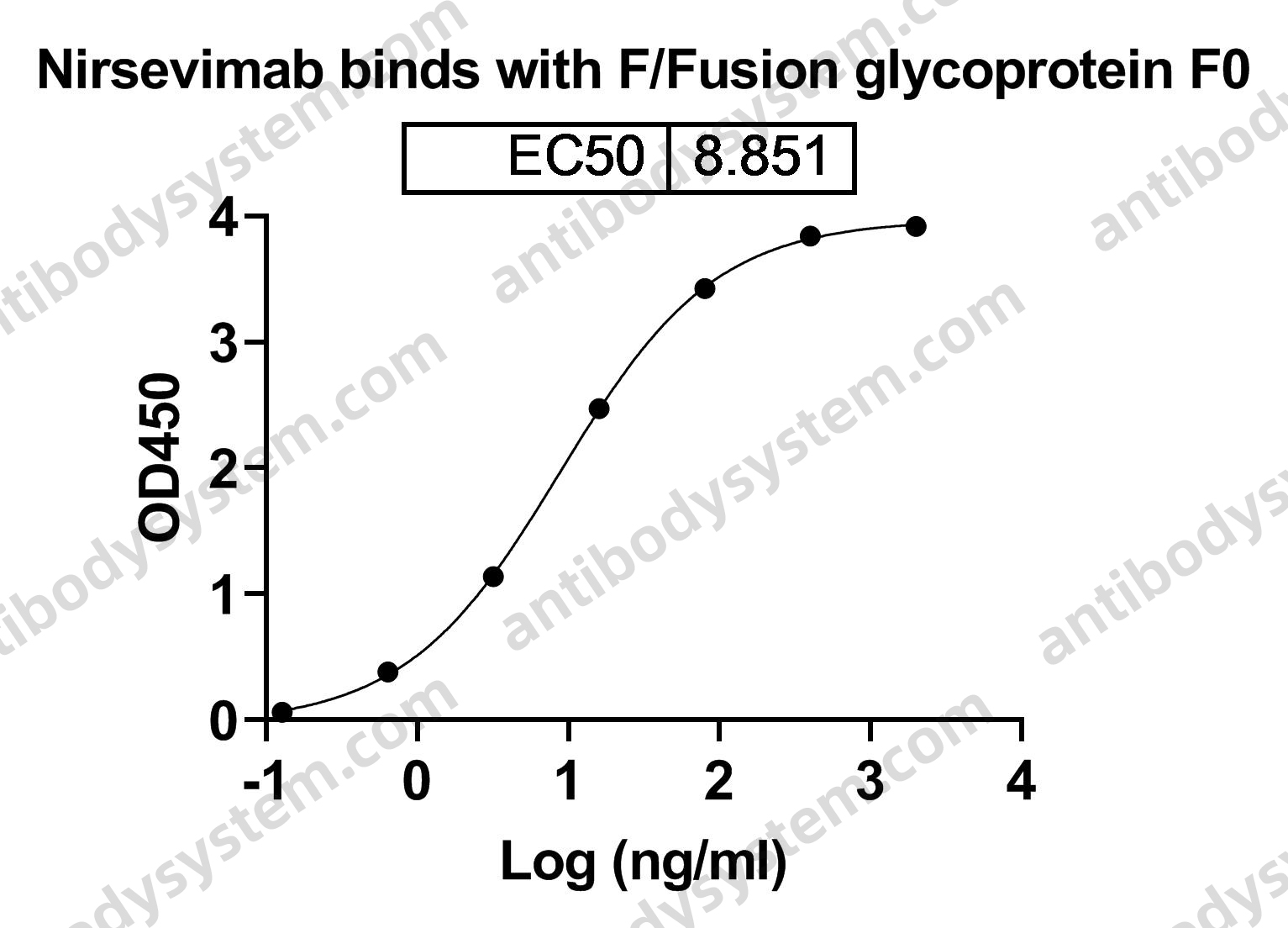
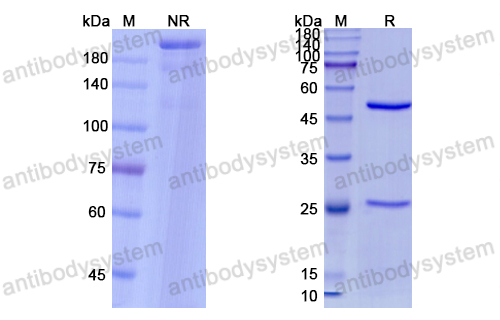
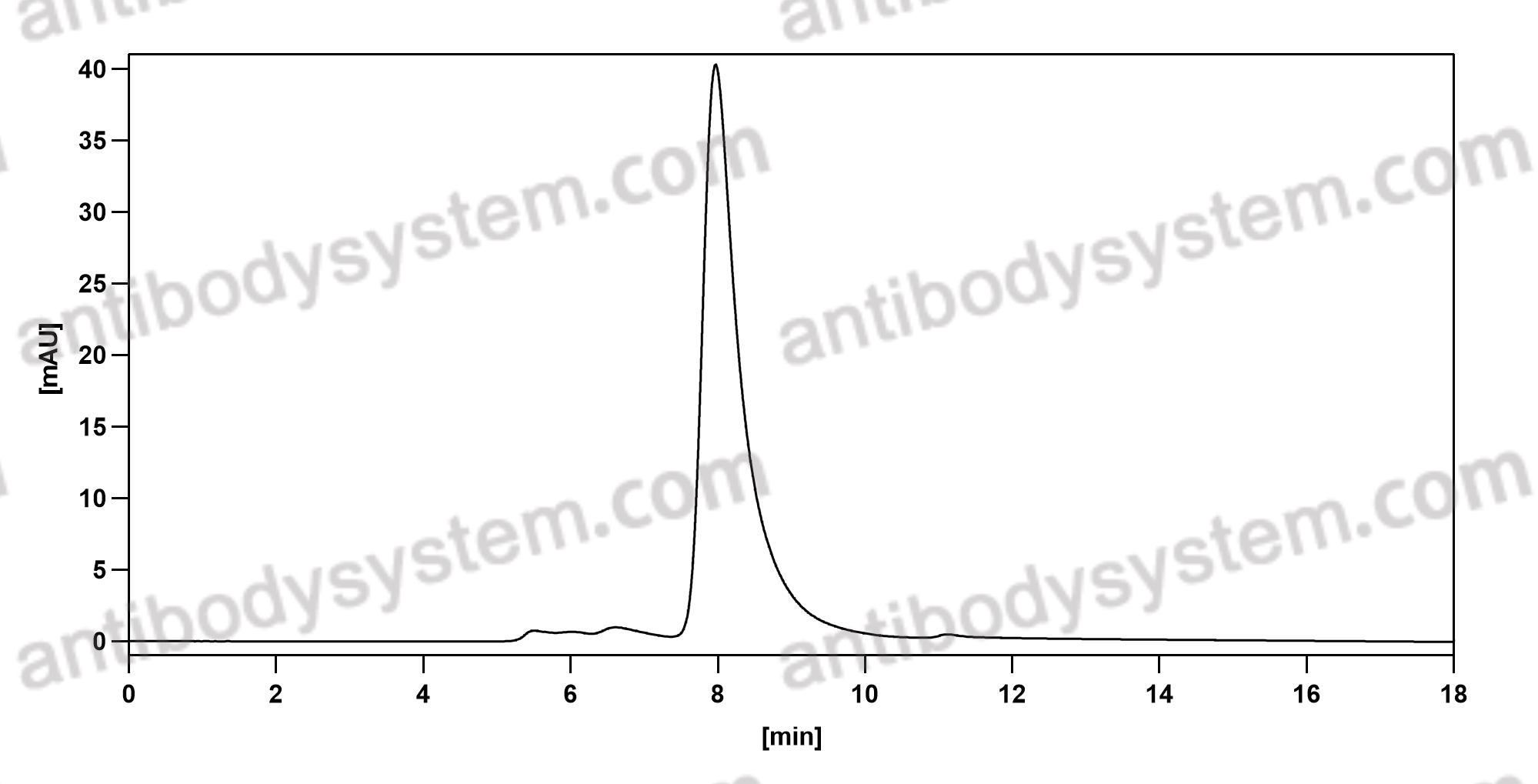
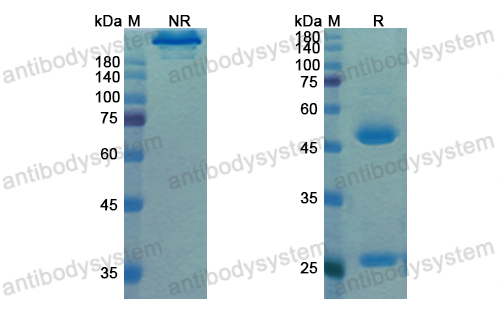
Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants, PMID: 32726528
Single-dose nirsevimab prevents RSV infection, PMID: 33342497
Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants, PMID: 32730699
Coadministration of Anti-Viral Monoclonal Antibodies With Routine Pediatric Vaccines and Implications for Nirsevimab Use: A White Paper, PMID: 34456918
Global molecular diversity of RSV - the "INFORM RSV" study, PMID: 32591017
Nirsevimab reduces medically attended RSV-associated lower respiratory tract infection and hospitalisations in healthy pre-term infants, PMID: 33597216
Respiratory Syncytial Virus and Human Metapneumovirus Infections in Three-Dimensional Human Airway Tissues Expose an Interesting Dichotomy in Viral Replication, Spread, and Inhibition by Neutralizing Antibodies, PMID: 32759319
Distinct patterns of within-host virus populations between two subgroups of human respiratory syncytial virus, PMID: 34446722
Development of a genetically modified full-length human respiratory syncytial virus preF protein vaccine. [DVV02802]
Real-world uptake of nirsevimab, RSV maternal vaccine, and RSV vaccines for older adults: a systematic review and meta-analysis., PMID:40524797
Will Nirsevimab Reduce the Burden on Public Health Care?-Reply., PMID:40522642
Will Nirsevimab Reduce the Burden on Public Health Care?, PMID:40522634
Effectiveness and impact of nirsevimab in Chile during the first season of a national immunisation strategy against RSV (NIRSE-CL): a retrospective observational study., PMID:40513593
Respiratory Syncytial Virus: A Narrative Review of Updates and Recent Advances in Epidemiology, Pathogenesis, Diagnosis, Management and Prevention., PMID:40507642
Nirsevimab: a change of paradigm in the prophylaxis of Respiratory Syncytial Virus disease among infants., PMID:40503651
Effectiveness of nirsevimab against RSV-related outcomes: findings of the 2024-2025 campaign in Catalonia align with previous analysis., PMID:40499942
Scenario Projections of Respiratory Syncytial Virus Hospitalizations Averted Due to New Immunizations., PMID:40498487
Evaluation of pregnant women's knowledge about RSV and immunization attitudes before infant immunization with monoclonal antibodies in Turkey., PMID:40489965
Demographic Characteristics Associated With Uptake of Neonatal Respiratory Syncytial Virus Prophylaxis., PMID:40489377
Strategies to improve interprofessional communication regarding maternal and infant RSV immunization., PMID:40483884
Nirsevimab Provides Real-World RSV Protection in Infants., PMID:40478586
Intention to Use RSVpreF Vaccine or Nirsevimab to Prevent Infant RSV Among Pregnant Individuals., PMID:40472256
Respiratory syncytial virus prophylaxis for children in Africa: Challenges, opportunities and public health strategies., PMID:40469396
Effectiveness of immunization strategies for preventing severe acute respiratory infection during the 2023/2024 season in a Spanish health department., PMID:40467410
Turkish pediatricians' knowledge, attitudes, and awareness of respiratory syncytial virus (RSV) infection and immunization strategies: a cross-sectional study., PMID:40466672
The potential public health impact of new immunization strategies for the prevention of RSV in children in Panama., PMID:40458968
Administration of Nirsevimab for RSV Prophylaxis in Infants: A Comprehensive Review., PMID:40432081
Impact of Nirsevimab immunoprophylaxis on professional exhaustion during two epidemics of respiratory syncytial virus., PMID:40414172
Vectored long-term co-delivery of antibodies for SARS-CoV-2, RSV and Influenza prophylaxis., PMID:40413832
Respiratory syncytial virus preventives for children in Australia: current landscape and future directions., PMID:40413643
Maternal Perspectives on Decision-Making for Newborn RSV Prophylaxis With Nirsevimab., PMID:40413636
Health-care burden related to respiratory syncytial virus in a resource-constrained setting: a prospective observational study., PMID:40412396
Estimated impact of nirsevimab prophylaxis on the economic burden of respiratory syncytial virus disease in Northern Italy., PMID:40400006
Cost-effectiveness analysis of nirsevimab for prevention of respiratory syncytial virus disease among infants in Shanghai, China: A modeling study., PMID:40391452
180-day efficacy of nirsevimab against hospitalisation for respiratory syncytial virus lower respiratory tract infections in infants (HARMONIE): a randomised, controlled, phase 3b trial., PMID:40379431
Update on Routine Immunizations for Children and Adolescents., PMID:40378322
Universal administration of nirsevimab in infants: an analysis of hospitalisations and paediatric intensive care unit admissions for RSV-associated lower respiratory tract infections., PMID:40377714
Direct Out-of-Pocket Costs of Nirsevimab vs. Palivizumab during the First Respiratory Syncytial Virus Season: A Comparative Analysis., PMID:40343422
RSV: an update on prevention and management., PMID:40343137
Estimating the impact of Western Australia's first respiratory syncytial virus immunisation program for all infants: A mathematical modelling study., PMID:40339485
Interim Evaluation of Respiratory Syncytial Virus Hospitalization Rates Among Infants and Young Children After Introduction of Respiratory Syncytial Virus Prevention Products - United States, October 2024-February 2025., PMID:40338822
Knowledge, attitudes, and practices of Croatian pediatricians and pediatrics residents about RSV infection., PMID:40328920
Infant Respiratory Syncytial Virus Immunization Coverage in the Vaccine Safety Datalink: 2023-2024., PMID:40324788
Real-world effectiveness of nirsevimab against respiratory syncytial virus disease in infants: a systematic review and meta-analysis., PMID:40319903
Cost-Effectiveness Analysis of Nirsevimab for the Prevention of Respiratory Syncytial Virus among Italian Infants., PMID:40317387
Respiratory syncytial virus-related lower respiratory tract infection hospitalizations in infants receiving nirsevimab in Galicia (Spain): the NIRSE-GAL study., PMID:40314706
Effectiveness of nirsevimab immunization against RSV infection in preterm infants: a systematic review and meta-analysis., PMID:40313952
Editorial: Surveillance of Seasonal Respiratory Syncytial Virus (RSV) Infection in Children and Vulnerable Adults Drives Vaccine Development and New Immunization Programs., PMID:40308086
Nirsevimab and Maternal Respiratory Syncytial Virus Vaccine Recommendations for the Pediatric Population., PMID:40305635
Impact of Nirsevimab on RSV and Non-RSV Severe Respiratory Infections in Hospitalized Infants., PMID:40302169
Nirsevimab immunisation of infants and respiratory syncytial virus (RSV)-associated hospitalisations, Western Australia, 2024: a population-based analysis., PMID:40293046
Nirsevimab prophylaxis on pediatric intensive care hospitalization for severe acute bronchiolitis: a clinical and economic analysis., PMID:40281363
Human iPS cell-derived respiratory organoids as a model for respiratory syncytial virus infection., PMID:40262853
Past, present and future of respiratory syncytial infection prevention in infants and young children., PMID:40243138
Global Pediatric Pulmonology Alliance recommendations to protect all infants against respiratory syncytial virus with prophylactic monoclonal antibodies., PMID:40241887
Summary of the National Advisory Committee on Immunization (NACI) Statement on the Prevention of Respiratory Syncytial Virus (RSV) in Infants., PMID:40241712
Navigating parental hesitancy in public health: the case for RSV immunization in newborns., PMID:40216995
Impact of routine prophylaxis with monoclonal antibodies and maternal immunisation to prevent respiratory syncytial virus hospitalisations, Lombardy region, Italy, 2024/25 season., PMID:40211969
Projecting maximum potential demand for nirsevimab to protect eligible US infants and young children against respiratory syncytial virus in the 2024/2025 season., PMID:40209626
Transamniotic Fetal Delivery of Recombinant Human Immunoglobulin Monoclonal Antibodies: A Potential Novel Strategy for Prevention of Neonatal Respiratory Syncytial Virus (RSV) Disease. [DVV02802]