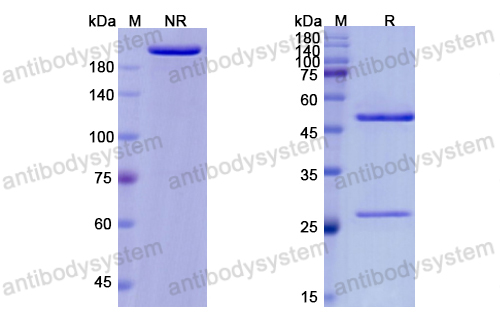
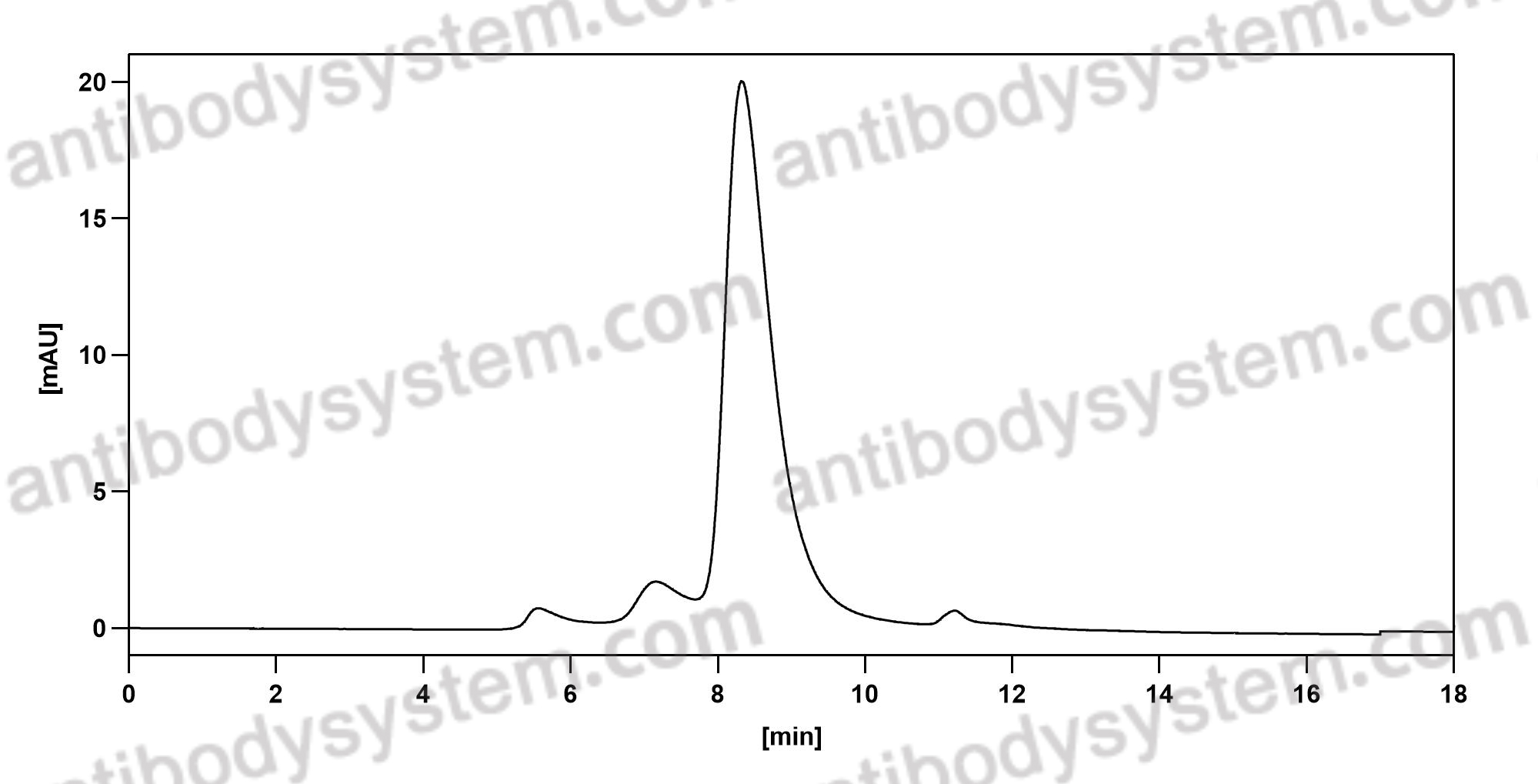
Ixekizumab for the treatment of psoriasis: up-to-date, PMID: 32050819
Ixekizumab, PMID: 28613740
Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis, PMID: 27299809
A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial, PMID: 32880909
Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial, PMID: 31813637
A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety and speed of response from a randomized, double-blinded trial, PMID: 31887225
Short-Term Efficacy and Safety of IL-17, IL-12/23, and IL-23 Inhibitors Brodalumab, Secukinumab, Ixekizumab, Ustekinumab, Guselkumab, Tildrakizumab, and Risankizumab for the Treatment of Moderate to Severe Plaque Psoriasis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials, PMID: 31583255
Ixekizumab for the treatment of ankylosing spondylitis, PMID: 32729361
Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial, PMID: 30360964
A systematic review and meta-analysis of the efficacy and safety of the interleukin (IL)-12/23 and IL-17 inhibitors ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab and tildrakizumab for the treatment of moderate to severe plaque psoriasis, PMID: 29532693
Ixekizumab, PMID: 31644231
[Ixekizumab], PMID: 31204120
Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial, PMID: 28551073
Drug survival of secukinumab and ixekizumab for moderate-to-severe plaque psoriasis, PMID: 30914343
Efficacy and safety of ixekizumab through 52 weeks in two phase 3, randomised, controlled clinical trials in patients with active radiographic axial spondyloarthritis (COAST-V and COAST-W), PMID: 31685553
The advent of IL-17A blockade in ankylosing spondylitis: secukinumab, ixekizumab and beyond, PMID: 30576610
Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1, PMID: 27553214
Ixekizumab for treating ankylosing spondylitis, PMID: 31530049
Ixekizumab for psoriasis, PMID: 29507458
A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial, PMID: 31563894
Ixekizumab, PMID: 29999864
Efficacy and Safety of Ixekizumab in the Treatment of Radiographic Axial Spondyloarthritis: Sixteen-Week Results From a Phase III Randomized, Double-Blind, Placebo-Controlled Trial in Patients With Prior Inadequate Response to or Intolerance of Tumor Necrosis Factor Inhibitors, PMID: 30343531
Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naïve to biological disease-modifying antirheumatic drug: final results by week 52, PMID: 32660977
Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials, PMID: 26072109
Ixekizumab Results in Persistent Clinical Improvement in Moderate-to-Severe Genital Psoriasis During a 52 Week, Randomized, Placebo-Controlled, Phase 3 Clinical Trial, PMID: 31620802
Paradoxical Reactions: Anti-Tumor Necrosis Factor Alpha Agents, Ustekinumab, Secukinumab, Ixekizumab, and Others, PMID: 29131037
Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS), PMID: 32316070
Efficacy and Safety of Ixekizumab Through 5 Years in Moderate-to-Severe Psoriasis: Long-Term Results from the UNCOVER-1 and UNCOVER-2 Phase-3 Randomized Controlled Trials, PMID: 32200512
The safety of ixekizumab in psoriasis drug therapy, PMID: 31874129
A review of ixekizumab in the treatment of psoriatic arthritis, PMID: 30360663
Ixekizumab in nail psoriasis, PMID: 32232895
Safety of ixekizumab in adult patients with plaque psoriasis, psoriatic arthritis and axial spondyloarthritis: data from 21 clinical trials, PMID: 32449924
Ixekizumab in the treatment of moderate-to-severe plaque psoriasis: Patient adherence, satisfaction, and preferences, PMID: 33135231
Ixekizumab for the treatment of psoriasis: an update on new data since first approval, PMID: 30589394
Ixekizumab provides superior efficacy compared with ustekinumab over 52 weeks of treatment: Results from IXORA-S, a phase 3 study, PMID: 29969700
Comparison of real-world treatment patterns among patients with psoriasis prescribed ixekizumab or secukinumab, PMID: 31712178
Ixekizumab: A Review of Its Use for the Management of Moderate to Severe Plaque Psoriasis, PMID: 30187769
Ixekizumab in the treatment of psoriatic arthritis, PMID: 33167745
Ixekizumab treatment of biologic-naïve patients with active psoriatic arthritis: 3-year results from a phase III clinical trial (SPIRIT-P1), PMID: 32031665
Safety of Ixekizumab Treatment for up to 5 Years in Adult Patients with Moderate-to-Severe Psoriasis: Results from Greater Than 17,000 Patient-Years of Exposure, PMID: 31749092
Comparison of short- and long-term effectiveness of ixekizumab and secukinumab in real-world practice, PMID: 33170052
Ixekizumab: First Global Approval, PMID: 27098317
Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: Results from a 52-week, open-label, phase 3 study (UNCOVER-J), PMID: 27726163
Ixekizumab and Ustekinumab Efficacy in Nail Psoriasis in Patients with Moderate-to-Severe Psoriasis: 52-Week Results from a Phase 3, Head-to-Head Study (IXORA-S), PMID: 32415575
Ixekizumab in genital psoriasis, PMID: 30318814
Long-term efficacy and safety of ixekizumab: A 5-year analysis of the UNCOVER-3 randomized controlled trial, PMID: 33253833
Ixekizumab: A Review in Moderate to Severe Plaque Psoriasis, PMID: 28138946
Ixekizumab: One drug for an enigmatic psoriasis, PMID: 32558074
Ixekizumab-induced alopecia areata, PMID: 31909139
Ixekizumab for treatment of psoriasis, PMID: 25748485
Rapid response to ixekizumab in a patient with erythrodermic psoriasis and drug-induced cirrhosis., PMID:40521057
Disease Modification in Psoriasis Through Early IL-17 Inhibitor Intervention: A Retrospective Cohort Study., PMID:40516878
Treatment of refractory pityriasis rubra pilaris with biologic therapy: a case series., PMID:40488550
Understanding Psoriasis Patient Preferences for Biologic Dosing Frequencies: Insights From a Patient Survey., PMID:40476147
A Case of Erythrodermic Psoriasis Successfully Treated With Ixekizumab Combined With Low-Dose Methotrexate to Ensure Sustained Clearance: A Case Report., PMID:40475334
Pityriasis rubra pilaris with symmetric polyarthritis and lymphadenopathy., PMID:40467080
Safety and efficacy of IL-17 inhibitors in patients with comorbid multiple sclerosis/multiple Sclerosis-like syndrome: a systematic review., PMID:40459586
Dissecting Cellulitis of the Scalp Successfully Treated with a Combination of Ixekizumab and Tofacitinib., PMID:40458693
The effect of IL-17 and IL-23 ınhibitors on hematological ınflammatory parameters in patients with psoriasis vulgaris., PMID:40455345
Psoriasis complicated with polymyositis successfully treated with Ixekizumab: A case report., PMID:40441209
Systematic review of comparative studies on emerging psoriasis treatments: comparing biologics with biologics, small molecule inhibitors with small molecule inhibitors, and biologics with small molecule inhibitors., PMID:40439875
Drug survival of IL-23 and IL-17 inhibitors versus other biologics for psoriasis: A British Association of Dermatologists Biologics and Immunomodulators Register cohort study., PMID:40439435
Paradoxical development of prurigo nodularis during ixekizumab therapy in a patient with generalized pustular psoriasis and psoriatic arthritis., PMID:40417893
Real-world safety assessment of Ixekizumab based on the FDA Adverse Event Reporting System (FAERS)., PMID:40408459
Real-World Utilization of Biologic and Targeted Synthetic Disease-Modifying Anti-rheumatic Drugs in Psoriatic Arthritis and Axial Spondyloarthritis: Insights from Sweden and Germany., PMID:40402376
Risk of adverse events of psoriasis treatment with biologic agents and new small molecules-BIOBADADERM Registry., PMID:40387427
Ixekizumab Retention Rate and Predictors of Treatment Persistence in Psoriatic Arthritis: Results of an Italian Multicenter Study., PMID:40376617
Extension of Secukinumab and Ixekizumab Dose for Moderate-To-Severe Psoriasis in Low Disease Activity Intervals., PMID:40369849
Thymic Bmi-1 hampers γδT17 generation and its derived RORγt-IL-17A signaling to delay cardiac aging., PMID:40366697
Efficacy of Ixekizumab in Chinese Patients with Radiographic Axial Spondyloarthritis by Baseline C-Reactive Protein Level., PMID:40343690
Trauma-Induced Psoriatic Arthritis: A Deep Köbner Phenomenon., PMID:40342834
Alopecia Areata Observed in a Patient Receiving Ixekizumab: A Case Report., PMID:40341668
Immunoproteomic Response to IL-17A Blockade in a Patient With Generalized Pustular Psoriasis: New Insights From Plasma Olink Profiling., PMID:40326672
Emerging manifestations of IL-17 immunomodulation in the gastrointestinal tract., PMID:40319948
Cutaneous and systemic improvements in psoriasis patients after different biologic treatments in a real-world longitudinal prospective study., PMID:40319105
Persistence of ixekizumab in psoriatic arthritis and the influence of gender., PMID:40315011
Ixekizumab demonstrates targeted protection against psoriatic arthritis in psoriasis patients: A multicenter retrospective cohort analysis., PMID:40288535
Efficacy and Safety of IL-17 and IL-23 Inhibitors in Elderly Patients With Plaque Psoriasis: A Real-World Study., PMID:40285439
Targeting nail psoriasis: IL-17A inhibitors demonstrate site-specific superiority over IL-23 inhibitor in a 24-week dermoscopy-guided real-world cohort., PMID:40264783
Case Report: Spesolimab for pyoderma gangrenosum in an undifferentiated oligoarthritis patient receiving anti-IL-17 therapy., PMID:40260260
[Successful subcutaneous desensitization in a patient with systemic reaction due to ixekizumab]., PMID:40253637
Unveiling the effectiveness and safety spectrum of biologic therapies in psoriasis: a three-year real-world analysis., PMID:40227059
BASDAI and ASDAS disease states in relationship to ASAS40 response: post hoc analysis of ixekizumab in radiographic axial spondyloarthritis., PMID:40201598
Chinese herbal medicine (Guben Qushi Huayu formula) combined with Ixekizumab in reducing psoriasis vulgaris relapse: Protocol for a mixed-methods research study., PMID:40191426
Treatment of Psoriasis with II-17 Inhibitors: Comparison of Long-Term Effectiveness and Drug Survival of Secukinumab vs Ixekizumab in Real-World Practice., PMID:40166484
The Efficacy, Safety and Longevity of Biologic Treatments in Pediatric and Adult Psoriasis Patients: A Comparative Multi-Center, Real-Life Study., PMID:40165569
Biological Disease-Modifying Antirheumatic Drugs Decrease Uric Acid Levels in the Sera of Patients with Psoriatic Arthritis., PMID:40136396
Small molecule interleukin (IL) 17A/A antagonists and antibodies blocking both IL17A/A and IL17A/F demonstrate equivalent degrees of efficacy in preclinical models of skin and joint inflammation., PMID:40127522
Hypopigmented mycosis fungoides-like eruption following ixekizumab treatment., PMID:40123792
A real-world study of ixekizumab use patterns, switching and efficacy in patients with plaque psoriasis., PMID:40119943
Risk of Herpes Zoster and Postherpetic Neuralgia in Patients with Psoriasis Treated with Biologics: A nationwide study using target trial emulation framework., PMID:40112174
Intra-Class Interleukin-(IL)-17 Blocker Switching: Ixekizumab as a Solution for Secukinumab Non-responders in Secondary Biologic Failure in Psoriasis., PMID:40092026
Safety and effectiveness of ixekizumab in Japanese patients with psoriasis vulgaris, psoriatic arthritis, generalized pustular psoriasis, and erythrodermic psoriasis: Post-marketing surveillance., PMID:40079483
A systematic review of the role of interleukin-17 inhibitors in bullous pemphigoid: therapeutic and paradoxical effects., PMID:40067544
Pityriasis Rubra Pilaris Following COVID-19 Infection: A Case of Successful Treatment With Ixekizumab., PMID:40062179
New and future perspectives in Behçet's syndrome., PMID:40060132
Baseline Pathological Liver Function Tests in Patients With Psoriasis Support the Indication for Systemic Therapy Rather Than Being a Reason Against It: A Real-World Analysis., PMID:40046951
Emerging treatments for dermatologic diseases in infants, children, and adolescents: a systematic review of clinical trials on biologics and small molecule inhibitors., PMID:40042725
Acute Generalized Exanthematous Pustulosis in a 76-year-Old Man With Neuroendocrine Carcinoma of the Lung, Responsive to Ixekizumab., PMID:40026376
Safety of Interleukin Inhibitors in Psoriatic Patients with Latent Tuberculosis Infection Without Chemoprophylaxis: A Systematic Review., PMID:40026108