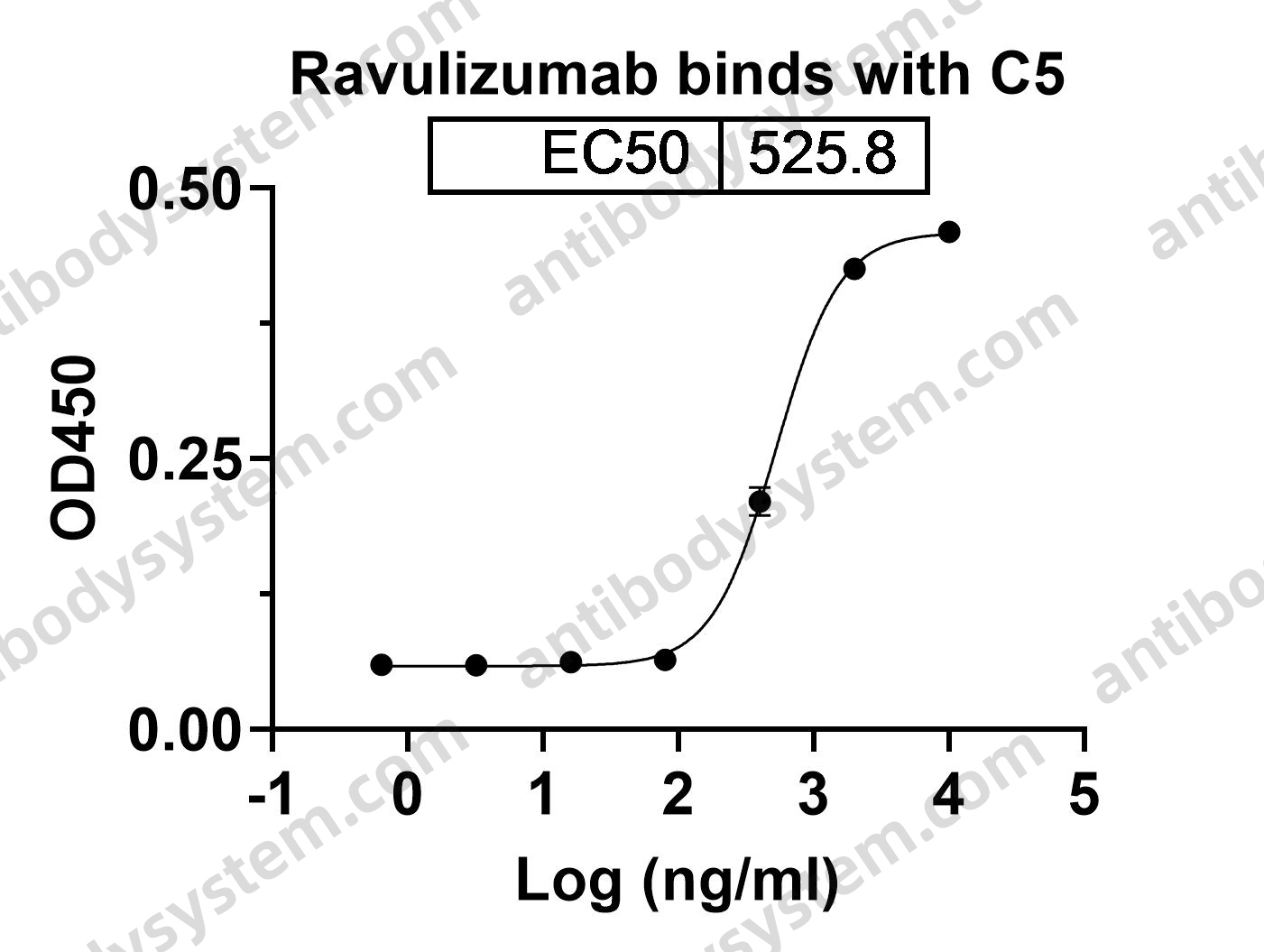
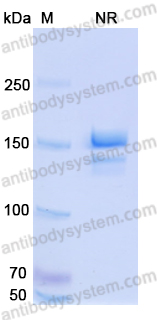
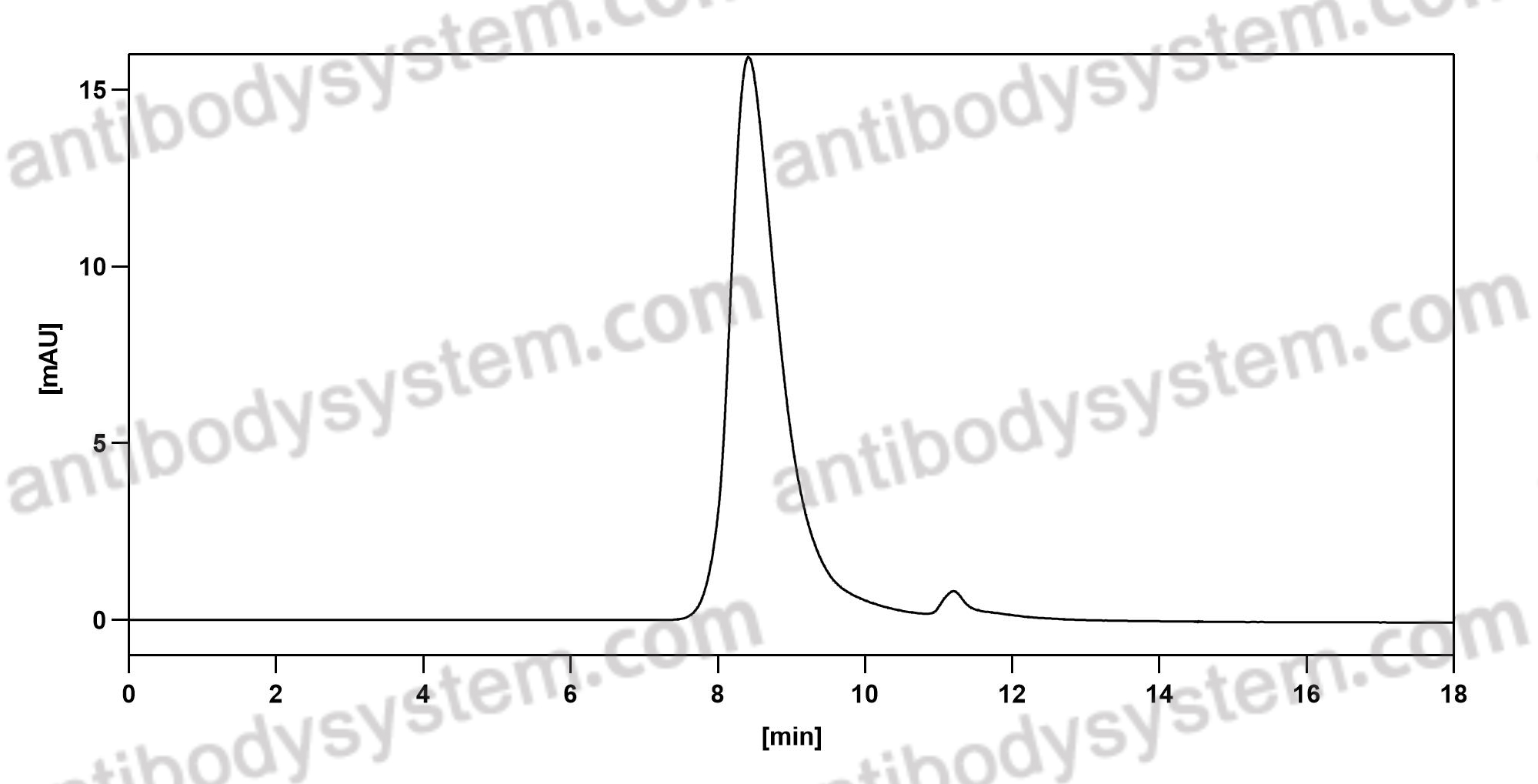
Ravulizumab: First Global Approval, PMID: 30767127
Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: the 302 study, PMID: 30510079
Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study, PMID: 30510080
Ravulizumab, PMID: 31643795
Ravulizumab (ALXN1210) in patients with paroxysmal nocturnal hemoglobinuria: results of 2 phase 1b/2 studies, PMID: 30171081
Ravulizumab, PMID: 30694619
Ravulizumab for the treatment of paroxysmal nocturnal hemoglobinuria, PMID: 32011183
The long-acting C5 inhibitor, ravulizumab, is efficacious and safe in pediatric patients with atypical hemolytic uremic syndrome previously treated with eculizumab, PMID: 33048203
Monitoring Ravulizumab effect on complement assays, PMID: 33321132
A Phase 3 Open-label, Randomized, Controlled Study to Evaluate the Efficacy and Safety of Intravenously Administered Ravulizumab Compared with Best Supportive Care in Patients with COVID-19 Severe Pneumonia, Acute Lung Injury, or Acute Respiratory Distress Syndrome: A structured summary of a study protocol for a randomised controlled trial, PMID: 32660611
The long-acting C5 inhibitor, Ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment, PMID: 32299680
Ravulizumab: a novel C5 inhibitor for the treatment of paroxysmal nocturnal hemoglobinuria, PMID: 31534662
Characterization of breakthrough hemolysis events observed in the phase 3 randomized studies of ravulizumab versus eculizumab in adults with paroxysmal nocturnal hemoglobinuria, PMID: 31949012
Pharmacokinetic and pharmacodynamic effects of ravulizumab and eculizumab on complement component 5 in adults with paroxysmal nocturnal haemoglobinuria: results of two phase 3 randomised, multicentre studies, PMID: 32449174
Ravulizumab: a complementary option for PNH, PMID: 30733201
Is ravulizumab the new treatment of choice for atypical hemolytic uremic syndrome (aHUS)?, PMID: 32444092
Ravulizumab: A Review in Atypical Haemolytic Uraemic Syndrome, PMID: 33738756
A US cost-minimization model comparing ravulizumab versus eculizumab for the treatment of atypical hemolytic uremic syndrome, PMID: 33001704
One-year efficacy and safety of ravulizumab in adults with paroxysmal nocturnal hemoglobinuria naïve to complement inhibitor therapy: open-label extension of a randomized study, PMID: 33178408
Patient preferences and quality of life implications of ravulizumab (every 8 weeks) and eculizumab (every 2 weeks) for the treatment of paroxysmal nocturnal hemoglobinuria, PMID: 32886668
No evidence for hypogammaglobulinemia in patients with paroxysmal nocturnal hemoglobinuria (PNH) chronically treated with ravulizumab, PMID: 32218584
Antibodies to watch in 2019, PMID: 30516432
Cost-Utility Analysis of Ravulizumab Compared with Eculizumab in Adult Patients with Paroxysmal Nocturnal Hemoglobinuria, PMID: 32519233
Results from multinational phase 3 studies of ravulizumab (ALXN1210) versus eculizumab in adults with paroxysmal nocturnal hemoglobinuria: subgroup analysis of Japanese patients, PMID: 32869125
New Therapeutic Targets and Treatment Options for Thrombotic Microangiopathy: Caplacizumab and Ravulizumab, PMID: 32715723
The long-acting C5 inhibitor, ravulizumab, is effective and safe in pediatric patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment, PMID: 33307104
Eculizumab and Beyond: The Past, Present, and Future of Complement Therapeutics, PMID: 31703946
Cost burden of breakthrough hemolysis in patients with paroxysmal nocturnal hemoglobinuria receiving ravulizumab versus eculizumab, PMID: 32856539
Emerging drugs for the treatment of neuromyelitis optica, PMID: 32731771
Anti-complement Treatment for Paroxysmal Nocturnal Hemoglobinuria: Time for Proximal Complement Inhibition? A Position Paper From the SAAWP of the EBMT, PMID: 31258525
Overview of current and emerging therapies for amytrophic lateral sclerosis, PMID: 32840332
One-year outcomes from a phase 3 randomized trial of ravulizumab in adults with paroxysmal nocturnal hemoglobinuria who received prior eculizumab, PMID: 33301613
Ravulizumab for the Treatment of aHUS in Adults: Improving Quality of Life, PMID: 34169186
Efficacy and safety of the long-acting C5 inhibitor ravulizumab in patients with atypical hemolytic uremic syndrome triggered by pregnancy: a subgroup analysis, PMID: 33407224
Switching From High-Dose Eculizumab to Ravulizumab in Paroxysmal Nocturnal Hemoglobinuria: A Case Report, PMID: 32803134
Disseminated gonococcal infection in a patient with paroxysmal nocturnal hemoglobinuria having received ravulizumab and meningococcal vaccine, PMID: 32624359
Pharmacokinetic and Pharmacodynamic Evaluation of Ravulizumab in Adults with Severe Coronavirus Disease 2019, PMID: 33826106
Erratum to "Rondeau E, Scully M, Ariceta G, Barbour T, Cataland S, Heyne N, Miyakawa Y, Ortiz S, Swenson E, Vallee M, Yoon S-S, Kavanagh D, Haller H; on behalf of the 311 Study Group. The long-acting C5 inhibitor, Ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment" Kidney Int. 2020;97:1287-1296, PMID: 33276869
Comparative effectiveness of pegcetacoplan versus ravulizumab in patients with paroxysmal nocturnal hemoglobinuria previously treated with eculizumab: a matching-adjusted indirect comparison, PMID: 34445916
Repurposed immunomodulatory drugs for Covid-19 in pre-ICu patients - mulTi-Arm Therapeutic study in pre-ICu patients admitted with Covid-19 - Repurposed Drugs (TACTIC-R): A structured summary of a study protocol for a randomised controlled trial, PMID: 32641154
Safety of current treatments for paroxysmal nocturnal hemoglobinuria, PMID: 33249943
Correction to: The long-acting C5 inhibitor, ravulizumab, is efficacious and safe in pediatric patients with atypical hemolytic uremic syndrome previously treated with eculizumab, PMID: 33296010
[Antibody therapy for paroxysmal nocturnal hemoglobinuria], PMID: 32908057
Pharmaceutical Approval Update, PMID: 30828229
The potential of individualized dosing of ravulizumab to improve patient-friendliness of paroxysmal nocturnal haemoglobinuria treatment at reduced costs, PMID: 33512711
Correction to: Ravulizumab: A Review in Atypical Haemolytic Uraemic Syndrome, PMID: 33871820
Correction to: Ravulizumab: A Review in Atypical Haemolytic Uraemic Syndrome, PMID: 34245447
Paroxysmal nocturnal hemoglobinuria: current treatments and unmet needs, PMID: 33356783
Long-Term Efficacy and Safety of the Long-Acting Complement C5 Inhibitor Ravulizumab for the Treatment of Atypical Hemolytic Uremic Syndrome in Adults, PMID: 34169200
Second-Generation C5 Inhibitors for Paroxysmal Nocturnal Hemoglobinuria, PMID: 31916226
Ocular Manifestations in Atypical Hemolytic Uremic Syndrome Treated With Ravulizumab: A Case Report and Review of the Literature., PMID:40530206
Patient-reported outcomes in patients with paroxysmal nocturnal hemoglobinuria treated with crovalimab and approved C5 inhibitors in the phase III COMMODORE 2 and 1 studies., PMID:40515823
Effectiveness and safety of ravulizumab for Japanese patients with atypical hemolytic uremic syndrome switched from eculizumab: an analysis of a post-marketing surveillance., PMID:40515788
Systemic Neisseria sicca Infection Associated With Ravulizumab Therapy in Myasthenia Gravis., PMID:40511556
Emerging Role of Targeted Monoclonal Antibodies in Neuromyelitis Optica Spectrum Disorders., PMID:40506618
Complement inhibitor therapy as a steroid-sparing strategy in generalized myasthenia gravis., PMID:40453104
A phase 3 study of ravulizumab to protect patients with chronic kidney disease from cardiac surgery-associated acute kidney injury and major adverse kidney events (ARTEMIS)., PMID:40448185
From checkboxes to emojis: a novel approach to patient-reported outcomes in multiple sclerosis., PMID:40447786
Oral iptacopan monotherapy in paroxysmal nocturnal haemoglobinuria: final 48-week results from the open-label, randomised, phase 3 APPLY-PNH trial in anti-C5-treated patients and the open-label, single-arm, phase 3 APPOINT-PNH trial in patients previously untreated with complement inhibitors., PMID:40447351
The Role of Complement in the Pathogenesis and Treatment of Myasthenia Gravis., PMID:40422242
Translating biomarker insights into practice: a path forward in TA-TMA management., PMID:40406401
Aggressive and Refractory Attack of AQP4-IgG-Positive Neuromyelitis Optica Spectrum Disorder Treated With Ravulizumab: A Case Report., PMID:40400837
PLASMIC score to aid diagnosis of aHUS: an analysis of C5 inhibitor clinical trials and the PINC AI™ healthcare database., PMID:40375186
Ravulizumab for generalized Myasthenia Gravis: a multicenter real-life experience., PMID:40366475
Advancements in Complement Inhibition for PNH and Primary Complement Mediated Thrombotic Microangiopathy., PMID:40354320
Clinical Features and Factors Associated With Outcome in Late Adult-Onset Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease., PMID:40324120
Real-World Case Series of Ravulizumab Use in Patients with Myasthenia Gravis in Romania., PMID:40309792
Assessing the symptom control provided by ravulizumab or efgartigimod in myasthenia gravis: an evaluation of the patient experience of two different treatment approaches., PMID:40293925
Anchored Indirect Treatment Comparison Finds Comparable Effects of Pegcetacoplan and Iptacopan in Paroxysmal Nocturnal Haemoglobinuria., PMID:40285403
Characterizing clinically significant extravascular hemolysis in adults with PNH on ravulizumab or eculizumab treatment., PMID:40273330
The Advancing Landscape of Paroxysmal Nocturnal Hemoglobinuria Treatment., PMID:40257298
Long-Term Efficacy and Safety of Ravulizumab in Adults With Anti-Acetylcholine Receptor Antibody-Positive Generalized Myasthenia Gravis: Final Results From the Phase 3 CHAMPION MG Open-Label Extension., PMID:40241307
Breakthrough hemolysis in paroxysmal nocturnal hemoglobinuria throughout clinical trials: from definition to clinical practice., PMID:40233322
Atypical Hemolytic Uremic Syndrome: A Review of Complement Dysregulation, Genetic Susceptibility and Multiorgan Involvement., PMID:40217974
Warren Alpert Medical School of Brown University: Clinicopathologic Conference: September 20th, 2024. A Woman in her 20s with Abdominal Pain, Anemia and Thrombocytopenia., PMID:40191706
[Unrelated bone marrow transplantation for acute myeloid leukemia evolved from paroxysmal nocturnal hemoglobinuria]., PMID:40175140
Ventral Subpial and Central Cord Enhancement in Spinal Neurosarcoidosis: The Reverse Trident Sign., PMID:40168633
Disseminated infection with Candida dubliniensis after ravulizumab and treatment with rezafungin - a case report., PMID:40120217
Evaluating Rituximab Failure Rates in Neuromyelitis Optica Spectrum Disorder: A Nationwide Real-World Study From South Korea., PMID:40065454
A Case of Atypical Hemolytic Uremic Syndrome With a Complement Factor I Mutation Triggered by a Femoral Neck Fracture., PMID:40001340
Real-world evidence of pegcetacoplan in patients with paroxysmal nocturnal haemoglobinuria: A nationwide Italian study., PMID:39985320
Ravulizumab and other complement inhibitors for the treatment of autoimmune disorders., PMID:39983521
The road ahead: emerging therapies for primary IgA nephropathy., PMID:39968279
Eculizumab or ravulizumab treatment effect in people with neuromyelitis optica spectrum disorder: a plain language summary of three studies., PMID:39936529
[New pathogenic treatments for myasthenia gravis]., PMID:39930674
Atheroembolic Kidney Disease and Atypical Hemolytic Uremic Syndrome: Two Sides of the Same Coin?, PMID:39929165
Thrombosis at Unusual Sites: Focus on Myeloproliferative Neoplasms and Paroxysmal Nocturnal Hemoglobinuria., PMID:39900098
Testing for myelin oligodendrocyte glycoprotein antibodies: Who, what, where, when, why, and how., PMID:39861933
Ravulizumab demonstrates long-term efficacy, safety and favorable patient survival in patients with paroxysmal nocturnal hemoglobinuria., PMID:39841198
Current status and perspectives of hematopoietic cell transplantation in patients with paroxysmal nocturnal hemoglobinuria., PMID:39840046
Pharmacokinetic characterization and exposure-response relationship of crovalimab in the COMMODORE 1, 2 and 3 and COMPOSER trials of patients with paroxysmal nocturnal haemoglobinuria., PMID:39835421
A Case of Neuromyelitis Optica Spectrum Disorder With Improvement in Urinary Retention After the Administration of Ravulizumab., PMID:39822442
C5 complement inhibition versus FcRn modulation in generalised myasthenia gravis., PMID:39798960
Complement inhibition in seropositive generalized myasthenia gravis as rescue therapy in impending and effective treatment in frequently recurring impending myasthenic crisis-a case series., PMID:39735401
Successful Control of Myasthenic Crisis After the Introduction of Ravulizumab in Myasthenia Gravis: A Case Report., PMID:39712723
Advances in Disease-Modifying Therapeutics for Chronic Neuromuscular Disorders., PMID:39708835
Long-term efficacy and safety of danicopan as add-on therapy to ravulizumab or eculizumab in PNH with significant EVH., PMID:39700502
Thymoma Associated Myasthenia Gravis Successfully Treated With Ravulizumab., PMID:39668649