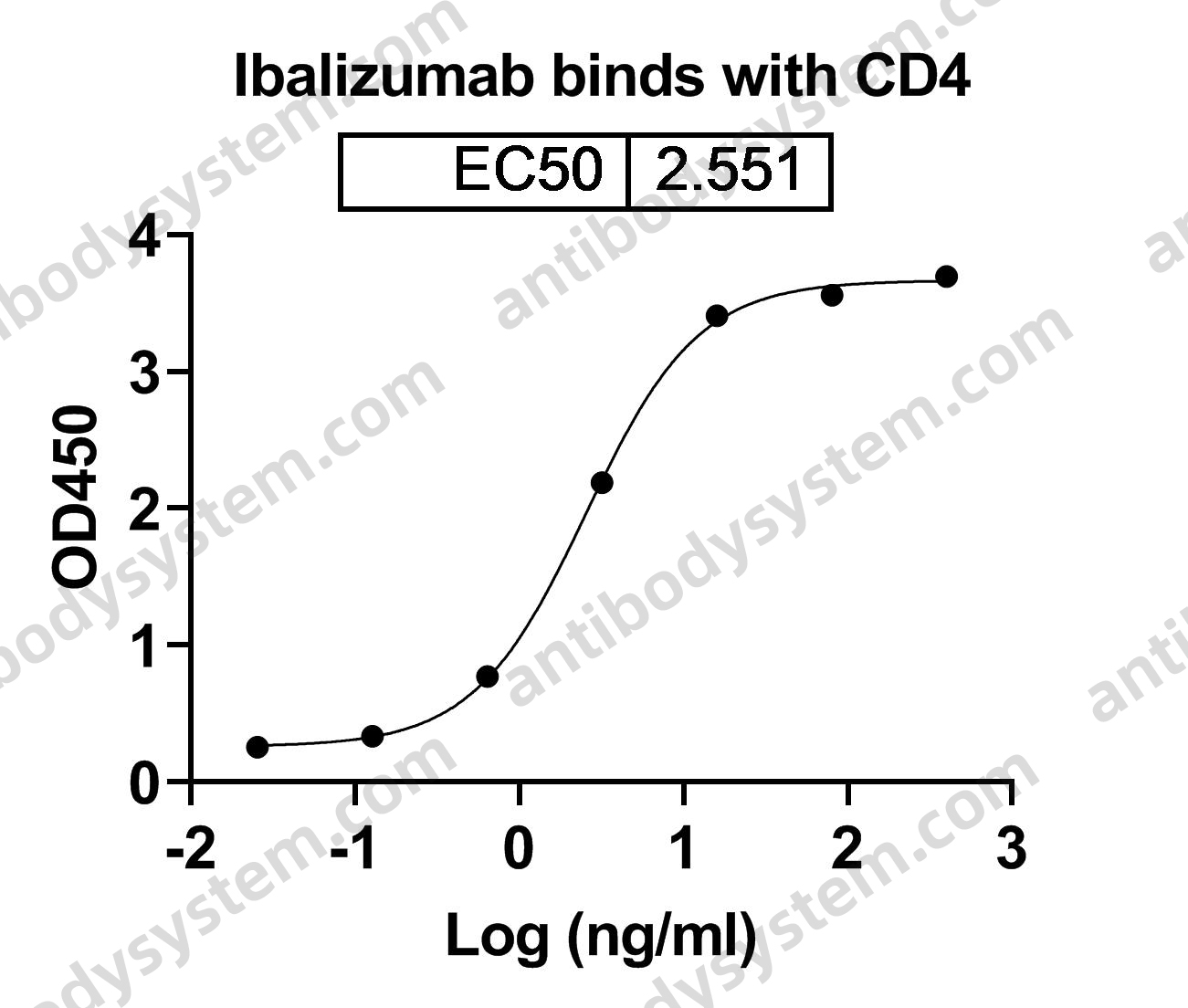
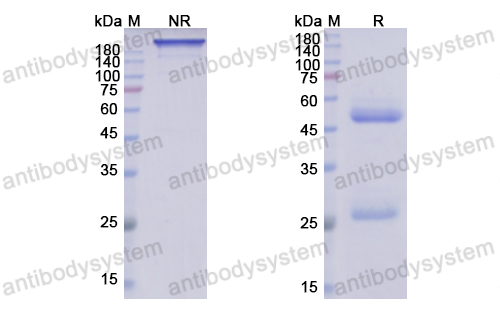
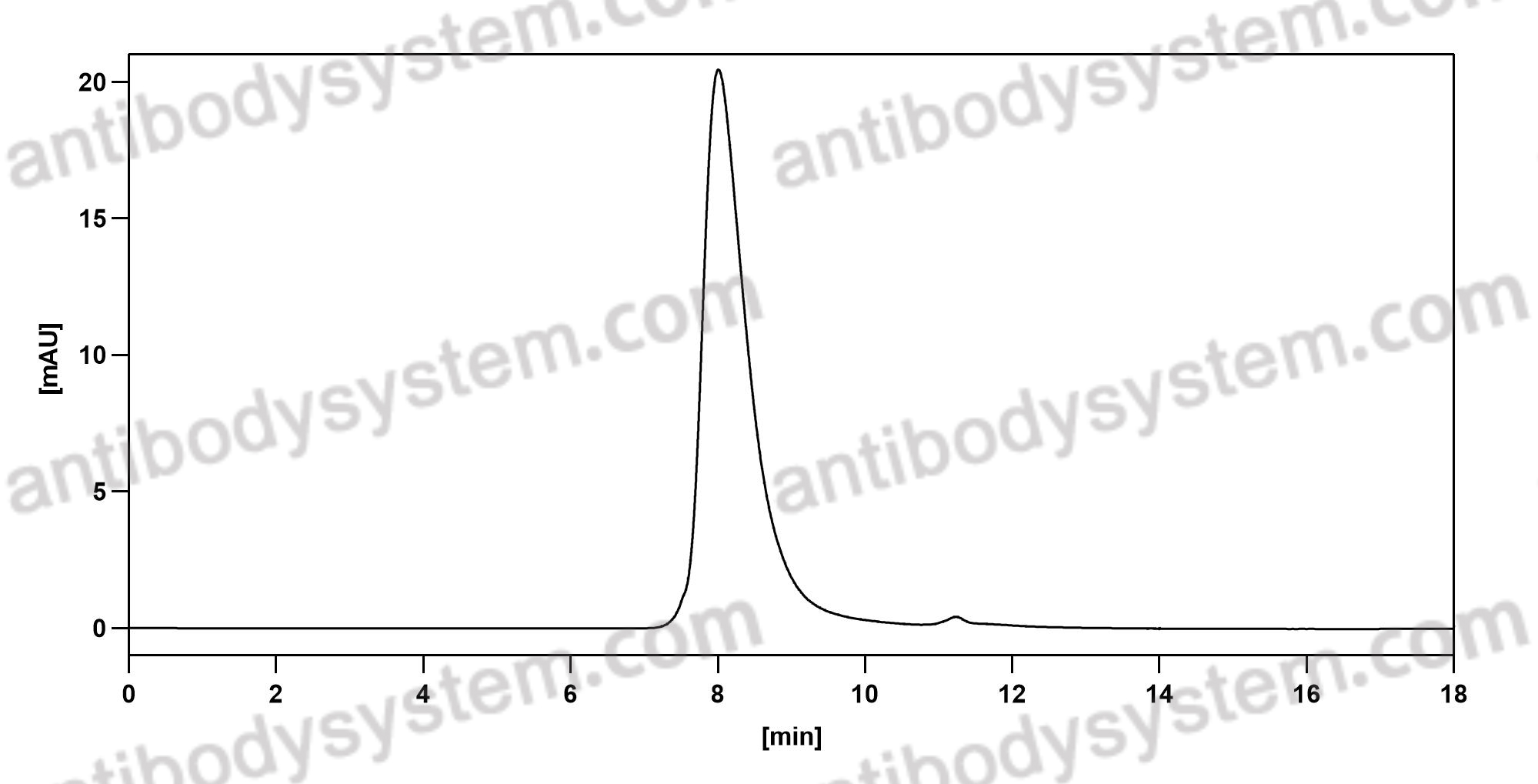
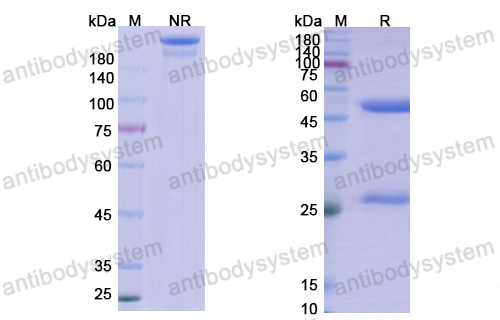
Ibalizumab, PMID: 29746266
Ibalizumab: A Review in Multidrug-Resistant HIV-1 Infection, PMID: 31970712
Ibalizumab, a Novel Monoclonal Antibody for the Management of Multidrug-Resistant HIV-1 Infection, PMID: 30885900
Ibalizumab: First Global Approval, PMID: 29675744
Phase 3 Study of Ibalizumab for Multidrug-Resistant HIV-1, PMID: 30110589
Ibalizumab, PMID: 31643628
Ibalizumab: The First Monoclonal Antibody for the Treatment of HIV-1 Infection, PMID: 32659101
Ibalizumab, PMID: 30000027
Ibalizumab and Fostemsavir in the Management of Heavily Pre-Treated HIV-infected Patients, PMID: 30378502
Ibalizumab for the treatment of multidrug-resistant HIV-1 infection, PMID: 30740610
Ibalizumab-uiyk (Trogarzo) for multidrug-resistant HIV, PMID: 29667947
Ibalizumab Targeting CD4 Receptors, An Emerging Molecule in HIV Therapy, PMID: 29230203
Ibalizumab in Multidrug-Resistant HIV - Accepting Uncertainty, PMID: 30110580
Cancer immunotherapy via targeted TGF-β signalling blockade in T H cells, PMID: 33087933
Bictegravir/emtricitabine/tenofovir alafenamide, Ibalizumab, and Ozenoxacin, PMID: 29954693
Clinical and Economic Impact of Ibalizumab for People With Multidrug-Resistant HIV in the United States, PMID: 31929403
Ibalizumab, a CD4-specific mAb to inhibit HIV-1 infection, PMID: 17668367
Correction to: Ibalizumab: First Global Approval, PMID: 29846911
Antibodies to watch in 2019, PMID: 30516432
Ibalizumab-human CD4 receptor interaction: computational alanine scanning molecular dynamics studies, PMID: 25756667
Efficacy, Pharmacokinetics, and Safety Over 48 Weeks With Ibalizumab-Based Therapy in Treatment-Experienced Adults Infected With HIV-1: A Phase 2a Study, PMID: 33427765
Insight into the modified Ibalizumab-human CD4 receptor interactions: using a computational binding free energy approach, PMID: 25342515
Epitope mapping of ibalizumab, a humanized anti-CD4 monoclonal antibody with anti-HIV-1 activity in infected patients, PMID: 20463063
Cerebrospinal fluid viral replication and burden of resistance in three HIV-1-infected people taking Ibalizumab with multiple drug class-wide resistance, PMID: 33105172
Anti-CD4 monoclonal antibody ibalizumab exhibits breadth and potency against HIV-1, with natural resistance mediated by the loss of a V5 glycan in envelope, PMID: 23023102
Loss of asparagine-linked glycosylation sites in variable region 5 of human immunodeficiency virus type 1 envelope is associated with resistance to CD4 antibody ibalizumab, PMID: 21289125
Ibalizumab: an anti-CD4 monoclonal antibody for the treatment of HIV-1 infection, PMID: 20639524
Safety, pharmacokinetics, and antiretroviral activity of multiple doses of ibalizumab (formerly TNX-355), an anti-CD4 monoclonal antibody, in human immunodeficiency virus type 1-infected adults, PMID: 19015347
Antibody-based strategies in HIV therapy, PMID: 33045349
Ibalizumab-uiyk as a bridge therapy for a patient with drug-resistant HIV-1 infection receiving chemotherapy: A case report, PMID: 34111306
The Cost-Effectiveness and Budget Impact of Ibalizumab-uiyk for Adults with Multidrug-Resistant HIV-1 Infection in the United States, PMID: 33532919
Strategic addition of an N-linked glycan to a monoclonal antibody improves its HIV-1-neutralizing activity, PMID: 24097413
Distinct HIV-1 Neutralization Potency Profiles of Ibalizumab-Based Bispecific Antibodies, PMID: 27792681
Anti-HIV-1 Antibodies: An Update, PMID: 32152957
Crystal structure of HIV-1 primary receptor CD4 in complex with a potent antiviral antibody, PMID: 21134642
Pharmaceutical Approval Update, PMID: 29896030
Next-generation oral preexposure prophylaxis: beyond tenofovir, PMID: 23032733
Choosing appropriate pharmacotherapy for drug-resistant HIV, PMID: 30676115
The efficacy of an anti-CD4 monoclonal antibody for HIV-1 treatment, PMID: 22001594
HIV-1 entry inhibitors: recent development and clinical use, PMID: 23290628
New Drugs 2019, part 2, PMID: 31022027
Generative models of conformational dynamics, PMID: 24446358
Recent advances in antiretroviral drugs, PMID: 20698725
HIV-1 entry inhibitors: an overview, PMID: 19339945
Antibodies to watch in 2018, PMID: 29300693
New drug for multidrug-resistant HIV, PMID: 29621221
Cross-species/cross-modality physiologically based pharmacokinetics for biologics: 89Zr-labelled albumin-binding domain antibody GSK3128349 in humans, PMID: 33073698
An option for treatment of multidrug-resistant HIV, PMID: 30194407
Current Status of the Pharmacokinetics and Pharmacodynamics of HIV-1 Entry Inhibitors and HIV Therapy, PMID: 28738768
Monoclonal Antibodies Against Infectious Microbes: So Long and Too Little!, PMID: 32164518
Ibalizumab plus an optimized background regimen in treatment-experienced patients infected with multidrug resistant HIV-1: A phase 3, multicenter, expanded access study., PMID:40465394
FlowDesign: Improved design of antibody CDRs through flow matching and better prior distributions., PMID:40306278
Investigating the combination of Temsavir and entry inhibitors on HIV replication: Synergistic and antagonistic effects observed against various R5-tropic envelopes., PMID:39642611
PRESTIGIO RING: "A 28-year-old highly treatmentexperienced man with vertical HIV infection on ibalizumab therapy: ART simplification perspectives"., PMID:39560045
Emerging integrase resistance in an international perinatal virtual clinic., PMID:39469738
Expanding Treatment Opportunities: Reviewing the Current State of Injectable Antiretrovirals for Treatment of HIV., PMID:39417932
Efficacy and Safety of 2 Fixed Doses of Ibalizumab Plus Optimized Background Regimen in Treatment-Experienced HIV-Positive Individuals., PMID:39250331
US cost-utility model of lenacapavir plus optimized background regimen (OBR) vs fostemsavir plus OBR and ibalizumab plus OBR for people with HIV with multidrug resistance., PMID:39213144
A Narrative Review of Novel Agents for Managing Heavily Treatment-Experienced People Living With HIV., PMID:39157636
Highly potent and broadly neutralizing anti-CD4 trimeric nanobodies inhibit HIV-1 infection by inducing CD4 conformational alteration., PMID:39138183
Strategic use of salvage long-acting antiretrovirals in the setting of resistance., PMID:39045845
Consensus recommendations for use of long-acting antiretroviral medications in the treatment and prevention of HIV-1: Endorsed by the American Academy of HIV Medicine, American College of Clinical Pharmacy, Canadian HIV and Viral Hepatitis Pharmacists Network, European AIDS Clinical Society, and Society of Infectious Diseases Pharmacists: An executive summary., PMID:39005161
Consensus recommendations for use of long-acting antiretroviral medications in the treatment and prevention of HIV-1: Endorsed by the American Academy of HIV Medicine, American College of Clinical Pharmacy, Canadian HIV and Viral Hepatitis Pharmacists Network, European AIDS Clinical Society, and Society of Infectious Diseases Pharmacists., PMID:39005160
Characterization of clinical envelopes with lack of sensitivity to the HIV-1 inhibitors temsavir and ibalizumab., PMID:38971430
Consensus recommendations for the use of novel antiretrovirals in persons with HIV who are heavily treatment-experienced and/or have multidrug-resistant HIV-1: Endorsed by the American Academy of HIV Medicine, American College of Clinical Pharmacy: An executive summary., PMID:38853605
Consensus recommendations for the use of novel antiretrovirals in persons with HIV who are heavily treatment-experienced and/or have multidrug-resistant HIV-1: Endorsed by the American Academy of HIV Medicine, American College of Clinical Pharmacy., PMID:38853601
Ex vivo sensitivity to broadly neutralizing antibodies and anti-CD4 antibody UB-421 of infectious viral isolates from people living with multidrug-resistant HIV., PMID:38728839
Salvage Therapy Including Foscarnet and Ibalizumab for Multidrug-Resistant Human Immunodeficiency Virus Type 2 Infection., PMID:38630945
Cross-resistance to entry inhibitors and lenacapavir resistance through Week 52 in study CAPELLA., PMID:38085652
Identification of potential therapeutic drugs targeting core genes for systemic lupus erythematosus (SLE) and coexisting COVID-19: Insights from bioinformatic analyses., PMID:38018597
Pharmacokinetics of Antiretroviral Drugs in Older People Living with HIV: A Systematic Review., PMID:37561283
Efficacy and Safety of Two-Drug Regimens That Are Approved from 2018 to 2022 for the Treatment of Human Immunodeficiency Virus (HIV) Disease and Its Opportunistic Infections., PMID:37374953
Lenacapavir (Sunlenca) for multidrug-resistant HIV., PMID:37155250
Indirect Treatment Comparisons of Lenacapavir Plus Optimized Background Regimen Versus Other Treatments for Multidrug-Resistant Human Immunodeficiency Virus., PMID:36566886
Realizing the promise of long-acting antiretroviral treatment strategies for individuals with HIV and adherence challenges: an illustrative case series., PMID:36435793
HIV-1 bispecific antibody iMab-N6 exhibits enhanced breadth but not potency over its parental antibodies iMab and N6., PMID:36071449
Quantitative PET imaging of the CD4 pool in nonhuman primates., PMID:36028577
Design of a Bispecific HIV Entry Inhibitor Targeting the Cell Receptor CD4 and Viral Fusion Protein Gp41., PMID:35711654
Comparative Efficacy and Safety of Fostemsavir in Heavily Treatment-Experienced People With HIV-1., PMID:35610081
Covalent binding of human two-domain CD4 to an HIV-1 subtype C SOSIP.664 trimer modulates its structural dynamics., PMID:35550505
Ibalizumab shows in-vitro activity against group A and group B HIV-2 clinical isolates., PMID:35262531
Achieving virological control in pan-resistant HIV-1 infection: A case series., PMID:35255457
Novel Bent Conformation of CD4 Induced by HIV-1 Inhibitor Indirectly Prevents Productive Viral Attachment., PMID:34896364
Clinical evidence for a lack of cross-resistance between temsavir and ibalizumab or maraviroc., PMID:34628442
Reactivation of Hepatitis B After Ibalizumab Therapy for Multidrug-Resistant Human Immunodeficiency Virus., PMID:34549064
Hepatotoxicity of contemporary antiretroviral drugs., PMID:34545037
Broad and potent bispecific neutralizing antibody gene delivery using adeno-associated viral vectors for passive immunization against HIV-1., PMID:34509584
Ibalizumab-uiyk as a bridge therapy for a patient with drug-resistant HIV-1 infection receiving chemotherapy: A case report., PMID:34111306
The Cost-Effectiveness and Budget Impact of Ibalizumab-uiyk for Adults with Multidrug-Resistant HIV-1 Infection in the United States., PMID:33532919
Efficacy, Pharmacokinetics, and Safety Over 48 Weeks With Ibalizumab-Based Therapy in Treatment-Experienced Adults Infected With HIV-1: A Phase 2a Study., PMID:33427765
Cerebrospinal fluid viral replication and burden of resistance in three HIV-1-infected people taking Ibalizumab with multiple drug class-wide resistance., PMID:33105172
Cancer immunotherapy via targeted TGF-β signalling blockade in TH cells., PMID:33087933
Cross-species/cross-modality physiologically based pharmacokinetics for biologics: 89Zr-labelled albumin-binding domain antibody GSK3128349 in humans., PMID:33073698
Antibody-based strategies in HIV therapy., PMID:33045349
Ibalizumab: The First Monoclonal Antibody for the Treatment of HIV-1 Infection., PMID:32659101
Monoclonal Antibodies Against Infectious Microbes: So Long and Too Little!, PMID:32164518
Anti-HIV-1 Antibodies: An Update., PMID:32152957
Ibalizumab: A Review in Multidrug-Resistant HIV-1 Infection., PMID:31970712
Clinical and Economic Impact of Ibalizumab for People With Multidrug-Resistant HIV in the United States., PMID:31929403