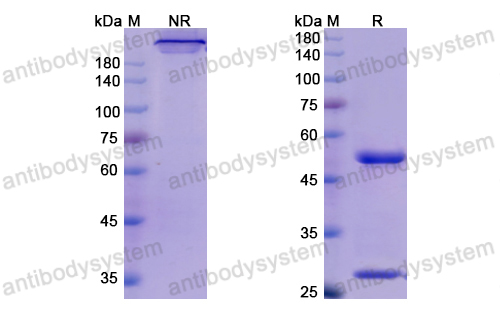
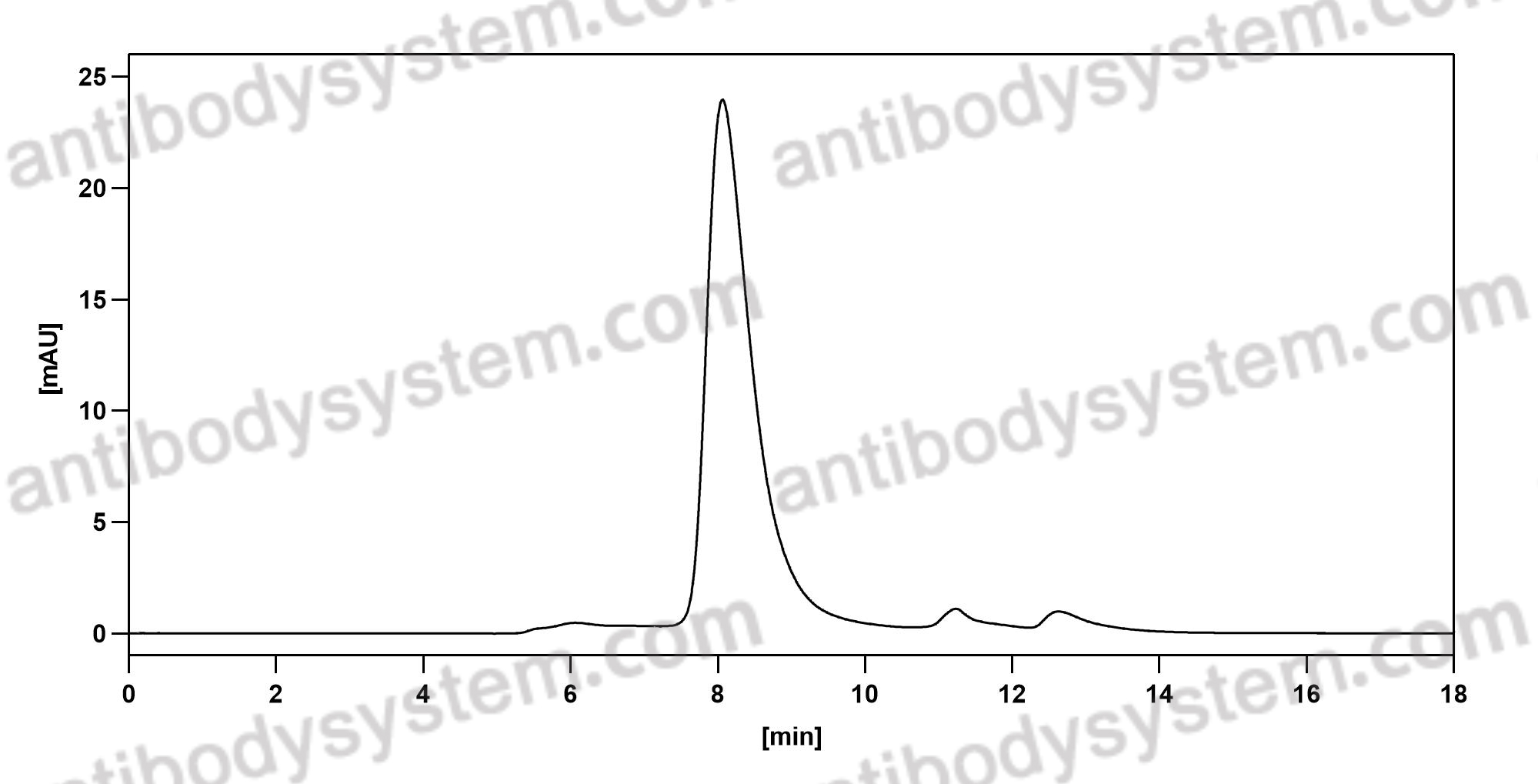
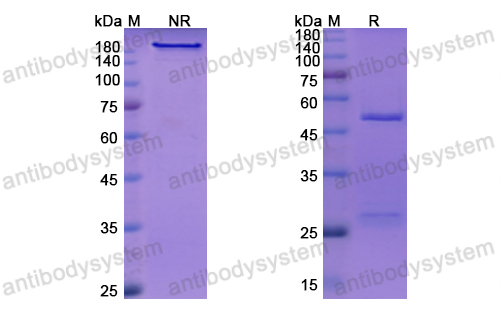
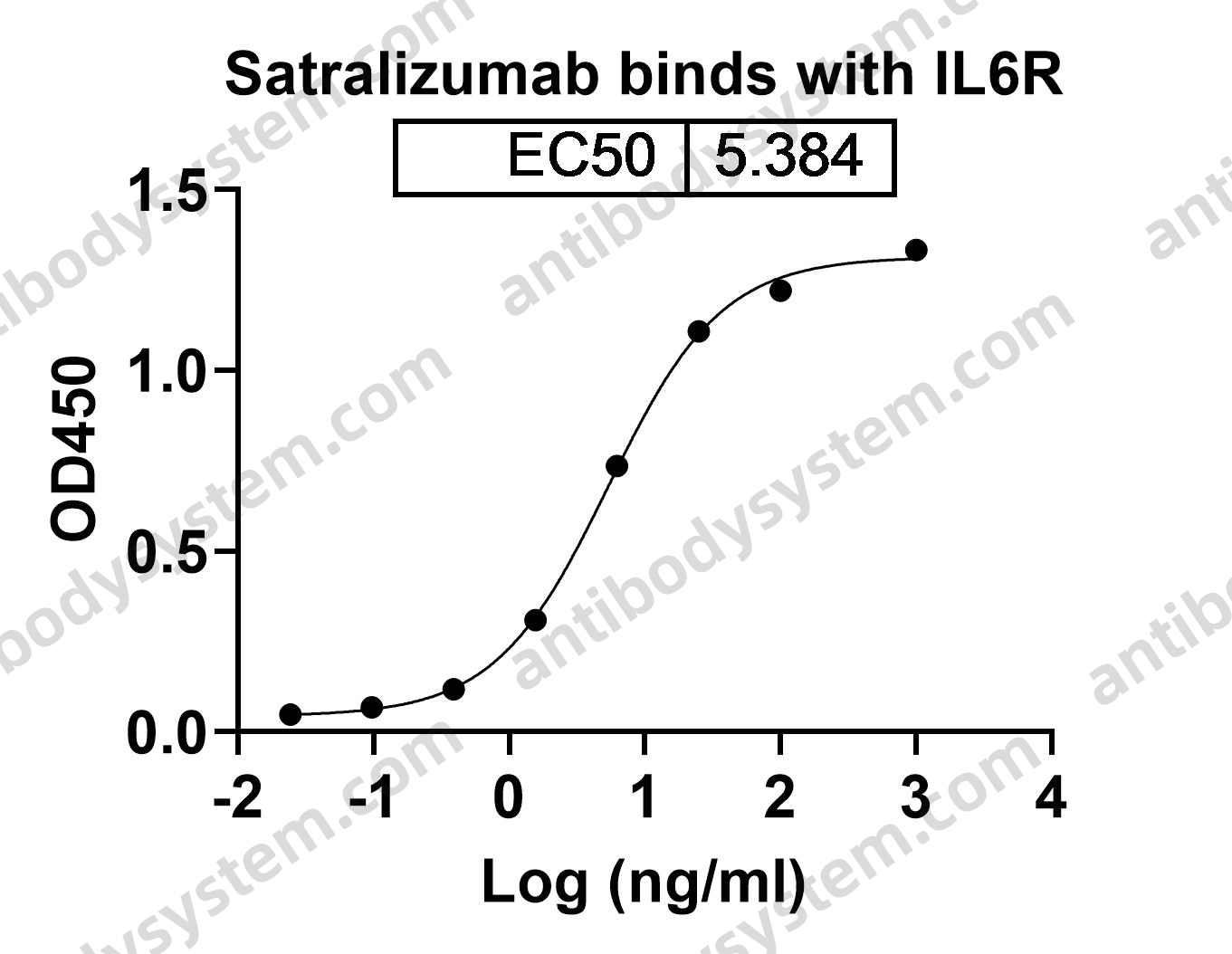
Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial, PMID: 30100375
Mogamulizumab for the Treatment of Adult T-cell Leukemia/Lymphoma, PMID: 30981611
Mogamulizumab: An Anti-CC Chemokine Receptor 4 Antibody for T-Cell Lymphomas, PMID: 31648540
Mogamulizumab, PMID: 31643268
Mogamulizumab, PMID: 30272896
Mogamulizumab in the treatment of advanced mycosis fungoides and Sézary syndrome: safety and efficacy, PMID: 32320304
Mogamulizumab: a new tool for management of cutaneous T-cell lymphoma, PMID: 30799938
Spotlight on Mogamulizumab-Kpkc for Use in Adults with Relapsed or Refractory Mycosis Fungoides or Sézary Syndrome: Efficacy, Safety, and Patient Selection, PMID: 32982179
Mogamulizumab for adult T-cell leukemia-lymphoma: a multicenter prospective observational study, PMID: 33091125
Mogamulizumab for the treatment of T-cell lymphoma, PMID: 28649848
Mogamulizumab-induced Mucocutaneous Lichenoid Reaction: A Case Report and Short Review, PMID: 32449779
Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: a randomized phase II study, PMID: 25733162
Mogamulizumab (Anti-CCR4) in HTLV-1-Associated Myelopathy, PMID: 29414279
Mogamulizumab-kpkc: A Novel Therapy for the Treatment of Cutaneous T-Cell Lymphoma, PMID: 33425472
A Phase I Study of the Anti-CC Chemokine Receptor 4 Antibody, Mogamulizumab, in Combination with Nivolumab in Patients with Advanced or Metastatic Solid Tumors, PMID: 31455681
Cutaneous T-Cell Lymphoma: Optimizing Care in Patients Receiving Anti-CCR4 Monoclonal Antibody Mogamulizumab, PMID: 31322628
Mogamulizumab in Combination with Durvalumab or Tremelimumab in Patients with Advanced Solid Tumors: A Phase I Study, PMID: 32586937
Histopathologic Characterization of Mogamulizumab-associated Rash, PMID: 32976123
Clinical efficacy of mogamulizumab for relapsed/refractory aggressive adult T-cell leukemia/lymphoma: A retrospective analysis, PMID: 32564395
Mogamulizumab Treatment Elicits Autoantibodies Attacking the Skin in Patients with Adult T-Cell Leukemia-Lymphoma, PMID: 31018922
A drug safety evaluation of mogamulizumab for the treatment of cutaneous T-Cell lymphoma, PMID: 31303060
Mogamulizumab for the treatment of adult T-cell leukemia/lymphoma, PMID: 23110261
Mogamulizumab versus investigator's choice of chemotherapy regimen in relapsed/refractory adult T-cell leukemia/lymphoma, PMID: 30573506
Mogamulizumab and the treatment of CCR4-positive T-cell lymphomas, PMID: 25496334
[Development of mogamulizumab and establishment of an optimal therapy based on genomic biomarkers: from the academic viewpoint], PMID: 25948299
Mogamulizumab for the treatment of relapsed or refractory adult T-cell leukemia-lymphoma, PMID: 28756726
A phase Ib study of utomilumab (PF-05082566) in combination with mogamulizumab in patients with advanced solid tumors, PMID: 31801624
Mogamulizumab Tops Standard of Care for CTCL, PMID: 29298770
Mogamulizumab for the treatment of cutaneous T-cell lymphoma: recent advances and clinical potential, PMID: 27247757
Mogamulizumab Plus EPOCH Therapy for Patients With Newly Diagnosed Aggressive Adult T-cell Leukemia/lymphoma, PMID: 32878812
Successful treatment of mogamulizumab-resistant mycosis fungoides with mogamulizumab plus etoposide combined therapy: Investigation of the immunomodulatory effects of etoposide on the tumor microenvironment, PMID: 32362053
Robust CD8+ T-cell proliferation and diversification after mogamulizumab in patients with adult T-cell leukemia-lymphoma, PMID: 32433748
Chemokines and Chemokine Receptors: New Targets for Cancer Immunotherapy, PMID: 30894861
Radiation recall dermatitis induced by mogamulizumab, PMID: 31660660
Mogamulizumab versus investigator choice in relapsed/refractory adult T-cell leukemia/lymphoma: all four one or none for all?, PMID: 31040231
NCCN Guidelines Insights: Primary Cutaneous Lymphomas, Version 2.2020, PMID: 32380458
Clinical significance of chemokine receptor antagonists, PMID: 31903790
Immunohistochemistry for CCR4 C-terminus predicts CCR4 mutations and mogamulizumab efficacy in adult T-cell leukemia/lymphoma, PMID: 33022137
Mogamulizumab Forecast: Clearer Patients, with a Slight Chance of Immune Mayhem, PMID: 31615932
Antibodies to watch in 2019, PMID: 30516432
Radiation-induced dermatitis after administration of mogamulizumab for adult T-cell leukaemia/lymphoma: a multi-institutional retrospective study, PMID: 30452692
Adult T-Cell Leukemia/Lymphoma, PMID: 28796966
Malignancy-associated pruritus, PMID: 26416212
CCR4 and its ligands: from bench to bedside, PMID: 25087232
Quality of Life Effect of the Anti-CCR4 Monoclonal Antibody Mogamulizumab Versus Vorinostat in Patients With Cutaneous T-cell Lymphoma, PMID: 33158772
FDA Approval Summary: Mogamulizumab-kpkc for Mycosis Fungoides and Sézary Syndrome, PMID: 31366601
Effects of mogamulizumab in adult T-cell leukemia/lymphoma in clinical practice, PMID: 28152225
How I treat adult T-cell leukemia/lymphoma, PMID: 33075812
Exposure-Response Analysis for Mogamulizumab in Adults With Cutaneous T-Cell Lymphoma, PMID: 31840837
Mogamulizumab-Associated Cutaneous Granulomatous Drug Eruption Mimicking Mycosis Fungoides but Possibly Indicating Durable Clinical Response, PMID: 31141114
Classical and biological treatments in mycosis fungoides/Sézary syndrome. New horizons in oncodermatology., PMID:40521064
Novel and recurrent histopathologic patterns of mogamulizumab-associated rash: diagnostic implications and insights for accurate diagnosis., PMID:40420651
Mogamulizumab induced lymph node enlargement mimicking mycosis fungoides progression., PMID:40392975
A phase 2 Trial of CHOP with Anti-CCR4 Antibody Mogamulizumab for older Patients with Adult T-Cell Leukemia/Lymphoma., PMID:40373281
Selecting appropriate therapy for cutaneous T-cell lymphomas (CTCLs): recent advances and the unmet need., PMID:40336318
Evaluation of the anti-CCR4 monoclonal antibody Mogamulizumab in advanced cutaneous T-cell lymphomas and with large cell transformation., PMID:40192548
Phase I study on neoadjuvant combination immunotherapy with mogamulizumab and nivolumab for solid tumors., PMID:40180420
Therapeutic advances for Cutaneous T Cell Lymphoma., PMID:40131851
Systemic Targeted Therapies in Patients with Relapsed/Refractory Advanced Stage Cutaneous T-cell Lymphoma: A Real-world Single-centre Case Series., PMID:40079767
Effects of tucidinostat in adult T-cell leukemia/lymphoma in clinical practice., PMID:40067419
Clinical and therapeutic significance of genetic profiling in adult T-cell leukemia/lymphoma., PMID:40056531
Cutaneous T-cell lymphomas: a real-life experience of anticipated use of mogamulizumab in Italy., PMID:40042219
[Successful treatment with EPOCH followed by mogamulizumab for angioimmunoblastic T-cell lymphoma with myelofibrosis and pure red cell aplasia]., PMID:39924203
Management of mycosis fungoides and Sézary syndrome with mogamulizumab in combination with psoralen plus UVA: two case reports., PMID:39906397
Mogamulizumab-induced alopecia. Multicentric case series: Clinical, trichoscopic and histological characterization., PMID:39902943
Significant detrimental impact of pre-transplant mogamulizumab on the post-transplant outcome with a short interval between the last mogamulizumab and transplantation., PMID:39901032
Insights into treatment of patients with mycosis fungoides or Sézary syndrome using mogamulizumab., PMID:39894454
The Progression of Mycosis Fungoides During Treatment with Mogamulizumab: A BIO-MUSE Case Study of the Tumor and Immune Response in Peripheral Blood and Tissue., PMID:39857770
Targeted antibody therapy as a treatment strategy for aggressive adult T-cell leukemia/lymphoma., PMID:39827571
Practical recommendations for therapy and monitoring of mogamulizumab patients in Germany., PMID:39723687
Successful Treatment of Mogamulizumab-Associated Rash With Upadacitinib: Evidence From 2 Cases., PMID:39661359
Mogamulizumab-Associated Autoimmune Diseases: Insights From FAERS Database Analysis., PMID:39659050
Clinical significance of NOTCH1 and FBXW7 alterations in adult T-cell leukemia/lymphoma., PMID:39586983
Potential mogamulizumab-associated inflammatory bowel disease in cutaneous T-cell lymphoma management., PMID:39583058
Improved survival among elderly patients with aggressive adult T-cell leukemia/lymphoma: Impact of mogamulizumab-containing chemotherapy., PMID:39390209
Real-world Use of Mogamulizumab Among Patients With Mycosis Fungoides and Sézary Syndrome Before and During COVID-19 in the United States., PMID:39379224
Chemotherapy and allo-HSCT for young patients with aggressive ATL., PMID:39366195
Mogamulizumab-induced bone granuloma., PMID:39332664
Mogamulizumab and Concomitant Hypofractionated Low-Dose Total Skin Electron Beam Therapy (2 × 4 Gy) in Cutaneous T-Cell Lymphoma: Proof of Principle, Report of Two Cases., PMID:39330028
Health-related quality of life in cutaneous T-cell lymphoma: A post hoc analysis of a phase 3 trial in mycosis fungoides and Sézary syndrome., PMID:39315857
A Retrospective Cohort Study to Determine Real-World Treatment Patterns in Patients with Sézary Syndrome in the United States., PMID:39305456
Systemic treatments with monoclonal antibodies in mycosis fungoides and Sézary syndrome., PMID:39295883
[Mogamulizumab-associated rash: cutaneous T-cell lymphoma in complete remission]., PMID:39278871
Atypical Presentation of Invasive Aspergillosis during Treatment with Mogamulizumab., PMID:39194909
Mogamulizumab for Sézary syndrome: long-term remission with associated autoimmune haemolytic anaemia., PMID:39193694
[Persistent Effects of Mogamulizumab on Peripheral Blood Lesions after Treatment Completion in a Patient with Refractory Sézary Syndrome-A Case Report]., PMID:39191715
A case report of refractory advanced-stage mycosis fungoides: successful treatment and improved patient quality of life with mogamulizumab., PMID:39091322
Real-life efficacy of immunotherapy for Sézary syndrome: a multicenter observational cohort study., PMID:39007062
Immune-related adverse events associated with mogamulizumab: a comprehensive review of the literature., PMID:38990648
Mogamulizumab-associated rash - Case series and review of the literature., PMID:38924340
Safety and effectiveness of mogamulizumab in relapsed or refractory CC chemokine receptor 4-positive peripheral T-cell lymphoma and relapsed or refractory cutaneous T-cell lymphoma: A post-marketing surveillance in Japan., PMID:38847317
Mogamulizumab associated crusted scabies: a rare diagnostic confounder., PMID:38842065
Advances in the pharmacological management of cutaneous T-cell lymphoma., PMID:38828644
Flow cytometric profiles with CD7 and CADM1 in CD4+ T cells are promising indicators for prognosis of aggressive ATL., PMID:38820467
A Narrative Review of the State of the Art of CCR4-Based Therapies in Cutaneous T-Cell Lymphomas: Focus on Mogamulizumab and Future Treatments., PMID:38804300
Effectiveness and safety of mogamulizumab for the treatment of Sézary syndrome in very elderly patients: A real-world, multicentre, retrospective study conducted in France., PMID:38804019
[Side effects of dermato-oncologic therapies]., PMID:38802653
[Drug-related exanthema under immunotherapy and targeted oncological therapy]., PMID:38772932
Fulminant hepatitis in a hepatitis B surface antigen-positive patient with adult T-cell leukemia-lymphoma after mogamulizumab monotherapy., PMID:38770705
Urticarial mycosis fungoides: A distinctive presentation with blood involvement and a peculiar immunophenotype., PMID:38769716