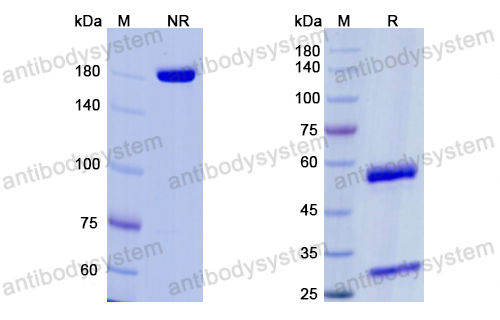
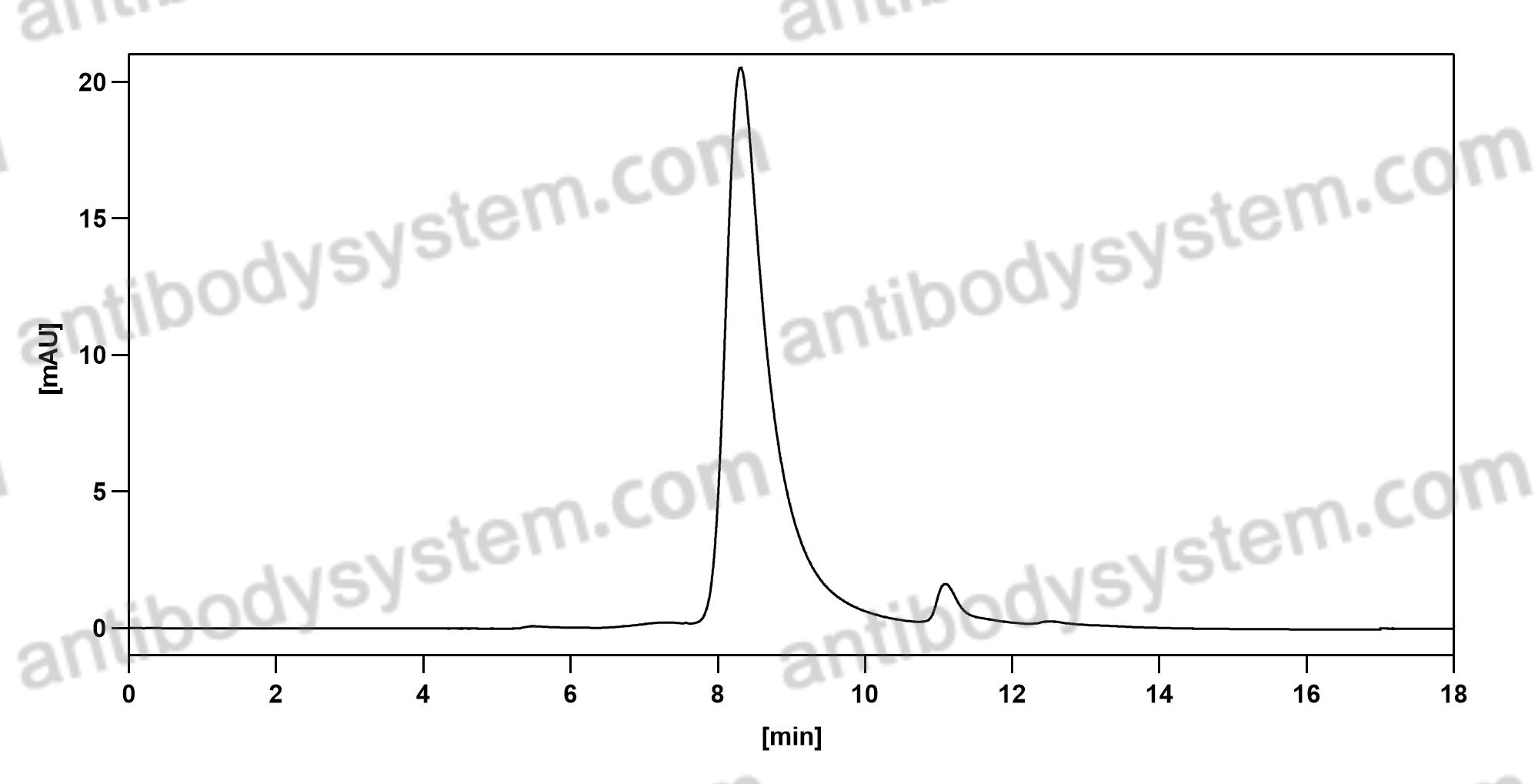
A Controlled Trial of Erenumab for Episodic Migraine, PMID: 29171821
Erenumab in the treatment of migraine, PMID: 30235976
Erenumab-aooe, PMID: 30559520
ARISE: A Phase 3 randomized trial of erenumab for episodic migraine, PMID: 29471679
The role of erenumab in the treatment of migraine, PMID: 32523630
Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial, PMID: 28460892
Erenumab for episodic migraine prophylaxis, PMID: 30614741
Vascular safety of erenumab for migraine prevention, PMID: 31852816
Erenumab in chronic migraine: Patient-reported outcomes in a randomized double-blind study, PMID: 30996060
Erenumab for migraine, PMID: 30670893
Erenumab: A First-in-Class Monoclonal Antibody for Migraine Prevention, PMID: 30813769
Erenumab safety and efficacy in migraine: A systematic review and meta-analysis of randomized clinical trials, PMID: 31876735
Erenumab: First Global Approval, PMID: 29968151
Early onset of efficacy with erenumab in patients with episodic and chronic migraine, PMID: 30276500
Real-World Patient Experience With Erenumab for the Preventive Treatment of Migraine, PMID: 32920850
Erenumab in chronic migraine with medication overuse: Subgroup analysis of a randomized trial, PMID: 30996056
Efficacy and safety of erenumab in women with a history of menstrual migraine, PMID: 32746775
Erenumab, PMID: 31643409
Nonclinical safety evaluation of erenumab, a CGRP receptor inhibitor for the prevention of migraine, PMID: 31085251
One-year sustained efficacy of erenumab in episodic migraine: Results of the STRIVE study, PMID: 32636324
Central effects of erenumab in migraine patients: An event-related functional imaging study, PMID: 32917805
Real-world effectiveness and tolerability of erenumab: A retrospective cohort study, PMID: 32791922
Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study, PMID: 30360965
Erenumab and galcanezumab in chronic migraine prevention: effects after treatment termination, PMID: 31159727
A Randomized Phase 2 Study of Erenumab for the Prevention of Episodic Migraine in Japanese Adults, PMID: 31612482
Erenumab, PMID: 30000991
Multidimensional assessment of the effects of erenumab in chronic migraine patients with previous unsuccessful preventive treatments: a comprehensive real-world experience, PMID: 32517693
Erenumab for Preventive Treatment of Migraine: A Systematic Review and Meta-Analysis of Efficacy and Safety, PMID: 30793254
In brief: Erenumab (Aimovig) hypersensitivity, PMID: 31022159
A prospective real-world analysis of erenumab in refractory chronic migraine, PMID: 32487102
Long-term safety and efficacy of erenumab in patients with chronic migraine: Results from a 52-week, open-label extension study, PMID: 32216456
Erenumab in Chronic Migraine: An Australian Experience, PMID: 32990364
Efficacy, tolerability, and safety of erenumab for the preventive treatment of persistent post-traumatic headache attributed to mild traumatic brain injury: an open-label study, PMID: 32493206
Migraine overview and summary of current and emerging treatment options, PMID: 30681821
Erenumab Side Effects, PMID: 31297805
Long-term efficacy and safety of erenumab in migraine prevention: Results from a 5-year, open-label treatment phase of a randomized clinical trial, PMID: 33400330
Erenumab-Induced Severe Nausea Leading to Smoking Cessation: A Retrospective Case Series, PMID: 33202039
Erenumab does not alter cerebral hemodynamics and endothelial function in migraine without aura, PMID: 32867533
Hypertension: A new safety risk for patients treated with erenumab, PMID: 33423274
Efficacy and safety of erenumab (AMG334) in chronic migraine patients with prior preventive treatment failure: A subgroup analysis of a randomized, double-blind, placebo-controlled study, PMID: 29984601
Erenumab During Breastfeeding, PMID: 31381367
Erenumab is effective in reducing migraine frequency and improving physical functioning, PMID: 30108057
Long-term safety and tolerability of erenumab: Three-plus year results from a five-year open-label extension study in episodic migraine, PMID: 31146544
Predicting erenumab adverse events with single-cell genomics, PMID: 32653068
Safety profile of erenumab, galcanezumab and fremanezumab in pregnancy and lactation: Analysis of the WHO pharmacovigilance database, PMID: 33435709
Pharmacoeconomic Review Report: Erenumab (Aimovig): (Novartis Pharmaceuticals Canada Inc.) [Internet], PMID: 33476110
Erenumab Efficacy on Comorbid Cluster Headache in Patients With Migraine: A Real-World Case Series, PMID: 32359106
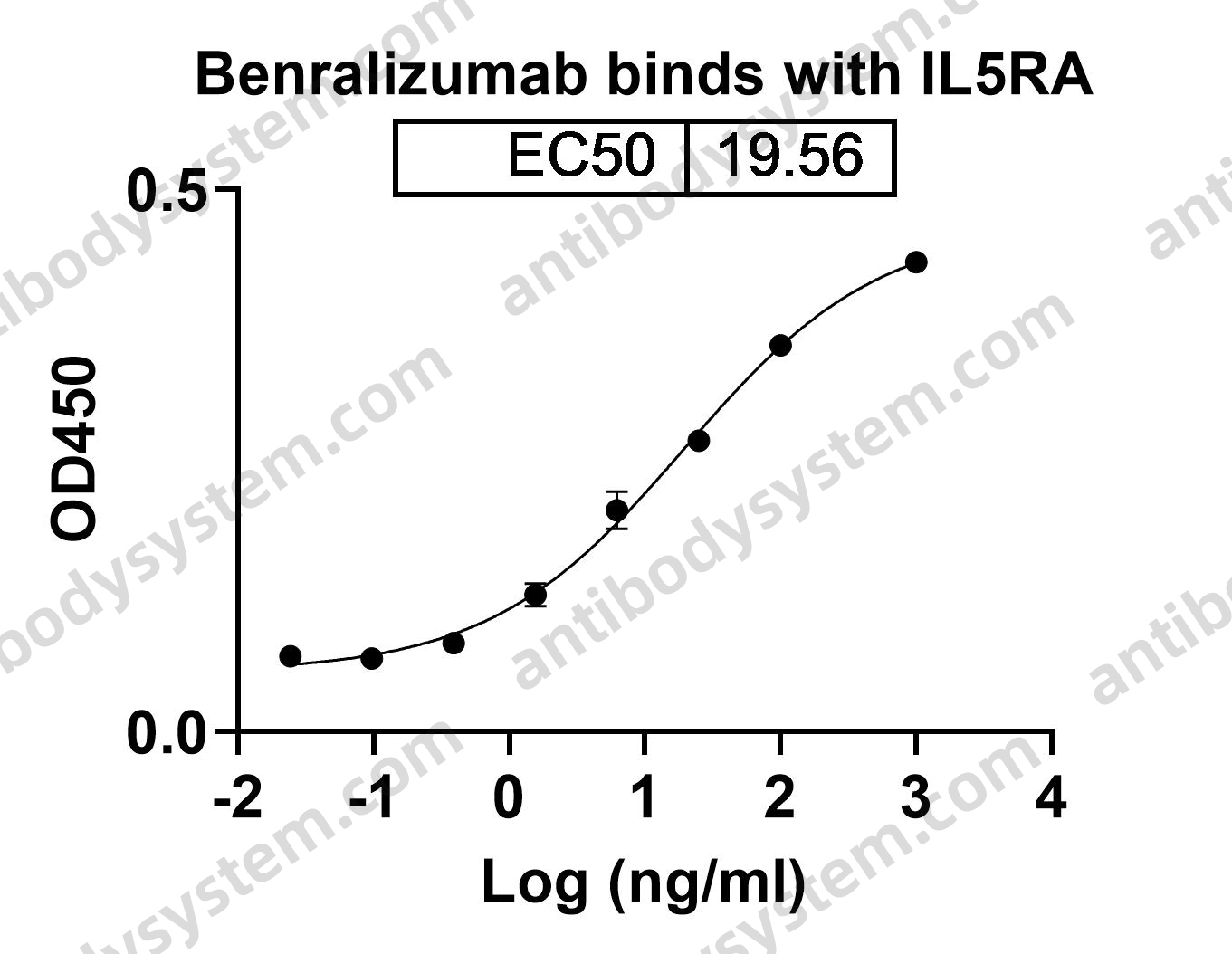
Erenumab-aooe, Benralizumab, and Tezacaftor/ivacaftor, PMID: 30190109
Erenumab in Chronic Migraine Patients Who Previously Failed Five First-Line Oral Prophylactics and OnabotulinumtoxinA: A Dual-Center Retrospective Observational Study, PMID: 32547474
Clinical Review Report: Erenumab (Aimovig): (Novartis Pharmaceuticals Canada Inc.) [Internet], PMID: 33471453
Erenumab for Chronic Cluster Headache: A Randomized Clinical Trial., PMID:40526384
Retrospective cohort study of anti-CGRP monoclonal antibody unresponsive migraine individuals treated with atogepant: The RESCUE study., PMID:40501126
Treatment of Persistent Headache After Normalization of CSF Pressure., PMID:40459314
Management of local and delayed cutaneous hypersensitivity reactions to anti-CGRP antibodies: implications for continuing treatment., PMID:40456979
Repurposed versus disease-specific medicinals for the prophylaxis of migraine: an updated systematic review., PMID:40447296
Postmarketing safety of migraine prophylactic monoclonal antibodies: An EudraVigilance database analysis of eptinezumab, fremanezumab, galcanezumab, and erenumab., PMID:40439253
Short-Term Immunosuppression in Rats Induces Prolonged Immune Tolerance Towards a Human Monoclonal Antibody, Erenumab., PMID:40379907
Calcitonin gene-related peptide-targeted therapies for migraine., PMID:40343132
Safety, efficacy, and compliance of moderate-to-high dose eptinezumab and erenumab in chronic migraine patients with medication-overuse headache: an updated systematic review and meta-analysis., PMID:40329188
Efficacy of monoclonal antibodies against CGRP in migraine patients with fibromyalgia comorbidity: a retrospective monocentric observational study., PMID:40329175
Swiss Quality of Life and Health Care Impact Assessment in a Real-World Erenumab-Treated Migraine Population: Results over 2 years., PMID:40304557
Increased infection risk in patients on preventive CGRP-targeting therapies- a meta-analysis and clinical effect assessment., PMID:40281424
Plasma SuPAR and therapeutic response to erenumab in migraine: a REFORM study., PMID:40275185
Clinical predictors for efficacy of erenumab for migraine: a Registry for Migraine (REFORM) study., PMID:40270925
Migraine treatment with CGRP monoclonal antibodies: patient evaluation of a mandatory treatment holiday in Belgium., PMID:40210844
[Successful Management of Wearing-off effect with Eptinezumab: Lessons from a case with Chronic Migraine Refractory to Two Subcutaneous CGRP Antibodies]., PMID:40191903
A National Cross-Sectional Survey on Real-World Experiences of Calcitonin Gene-Related Peptide (CGRP) Monoclonal Antibody Use in Adults with Migraine in Finland., PMID:40189729
Efficacy and safety of mAbs anti-CGRP/CGRP R (eptinezumab and erenumab) or atogepant in combination with onabotulinumtoxinA in refractory chronic migraine: a clinical trial protocol., PMID:40169386
Quickest way to less headache days: an operational research model and its implementation for chronic migraine., PMID:40165130
Health care resource utilization and direct costs incurred over 12 months by patients with migraine initiating self-injectable calcitonin gene-related peptide monoclonal antibodies: A US real-world study., PMID:40152794
Tracking Migraine Symptoms: A Longitudinal Comparison of Smartphone-Based Headache Diaries and Clinical Interviews., PMID:40137454
Patient satisfaction with calcitonin gene-related peptide monoclonal antibodies in migraine: A multicenter prospective cohort study., PMID:40135519
Unveiling the efficacy and safety of Erenumab, a monoclonal antibody targeting calcitonin gene-related peptide (CGRP) receptor, in patients with chronic and episodic migraine: a GRADE-assessed systematic review and meta-analysis of randomized clinical trials with subgroup analysis., PMID:40134001
CROATIAN GUIDELINES FOR SPECIFIC PREVENTIVE TREATMENT OF MIGRAINE WITH MONOCLONAL ANTIBODIES TARGETING CALCITONIN GENE-RELATED PEPTIDE (CGRP) (EPTINEZUMAB, FREMANEZUMAB, AND GALCANEZUMAB) OR THE CGRP RECEPTOR (ERENUMAB)., PMID:40104234
Ubrogepant, erenumab, and eptinezumab antagonize positive inotropic effects of the calcitonin gene-related peptide in the isolated human atrium., PMID:40085216
Assessing blood pressure changes and hypertension-related outcomes in patients with migraine treated with erenumab: A systematic review and meta-analysis., PMID:40084674
Efficacy and continuability of 675 mg fremanezumab administration over 2 years., PMID:40069630
Treatment Patterns and Healthcare Resource Utilization by Gender and Migraine Frequency in Adult Patients Receiving Galcanezumab Versus Standard of Care Preventive Medications Over 24 months: A United States Retrospective Claims Study., PMID:40046564
Effect of erenumab versus other migraine preventive medications on cardiovascular and cerebrovascular outcomes: A United States claims database-based observational cohort study., PMID:40033803
Real-world adherence to erenumab, rescue medication utilization, and work absenteeism for patients with migraine: Results from an outcomes-based agreement., PMID:40021466
Real-World Lessons with Fremanezumab as the Third Available CGRP Monoclonal Antibody in a Third-Level Hospital: Focus on the Factors Predicting Response., PMID:40004586
Review: An Update on CGRP Monoclonal Antibodies for the Preventive Treatment of Episodic Migraine., PMID:39998706
Health Technology Assessment: Evaluation of 8 CGRP-Targeted Therapy Drugs for the Treatment of Migraine., PMID:39991088
The effect of calcitonin gene-related peptide monoclonal antibodies on restless legs syndrome in patients with migraine., PMID:39972460
Popliteal Artery Rupture: A Rare but Life-Threatening Complication of Total Knee Arthroplasty., PMID:39935650
Improvement of Prurigo Nodularis With Erenumab., PMID:39908053
Signs of Cortical Inflammation in Migraine Measured with Quantitative Magnetic Resonance Imaging: A Registry for Migraine (REFORM) Study., PMID:39902552
Prevention of Episodic Migraine Headache Using Pharmacologic Treatments in Outpatient Settings: A Clinical Guideline From the American College of Physicians., PMID:39899861
Paralytic Ileus Following Long-term Erenumab Treatment., PMID:39894489
Switching between anti-CGRP monoclonal antibodies in migraine prophylaxis., PMID:39884968
Erenumab in a patient with persistent headaches after subarachnoid hemorrhage: A case report of an effective treatment., PMID:39865710
Cost-Utility Analysis of Erenumab Compared to Topiramate for Preventive Therapy of Migraine in Iran., PMID:39830656
Pharmacological differences and switching among anti-CGRP monoclonal antibodies: A narrative review., PMID:39825578
Efficacy and Safety of Switching Between Anti-CGRP Monoclonal Antibodies: A Detailed Monthly and Long-term Follow-up Study and Literature Review., PMID:39756881
Comparison of Early-onset Efficacy of Anti-calcitonin Gene-related Peptide Monoclonal Antibodies for Patients with Migraine in Real-world Clinical Practice: Study Protocol for an Exploratory Clinical Trial., PMID:39662907
Switching from ligand to receptor anti-calcitonin gene-related peptide (CGRP) antibodies or vice versa in non-responders: A controlled cohort study., PMID:39607215
Benefit-risk assessment based on number needed to treat and number needed to harm: Atogepant vs. calcitonin gene-related peptide monoclonal antibodies., PMID:39558612
Worldwide availability of medications for migraine and tension-type headache: A survey of the International Headache Society., PMID:39552307
Crowdsourcing Adverse Events Associated With Monoclonal Antibodies Targeting Calcitonin Gene-Related Peptide Signaling for Migraine Prevention: Natural Language Processing Analysis of Social Media., PMID:39515814
Efficacy of eptinezumab in non-responders to subcutaneous monoclonal antibodies against CGRP and the CGRP receptor: A retrospective cohort study., PMID:39469839