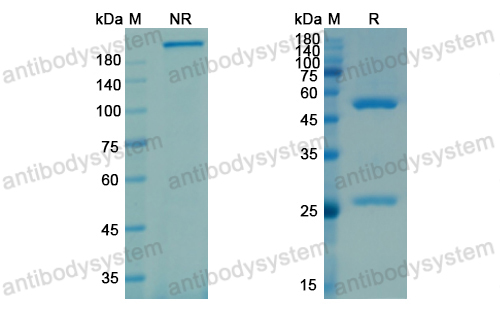
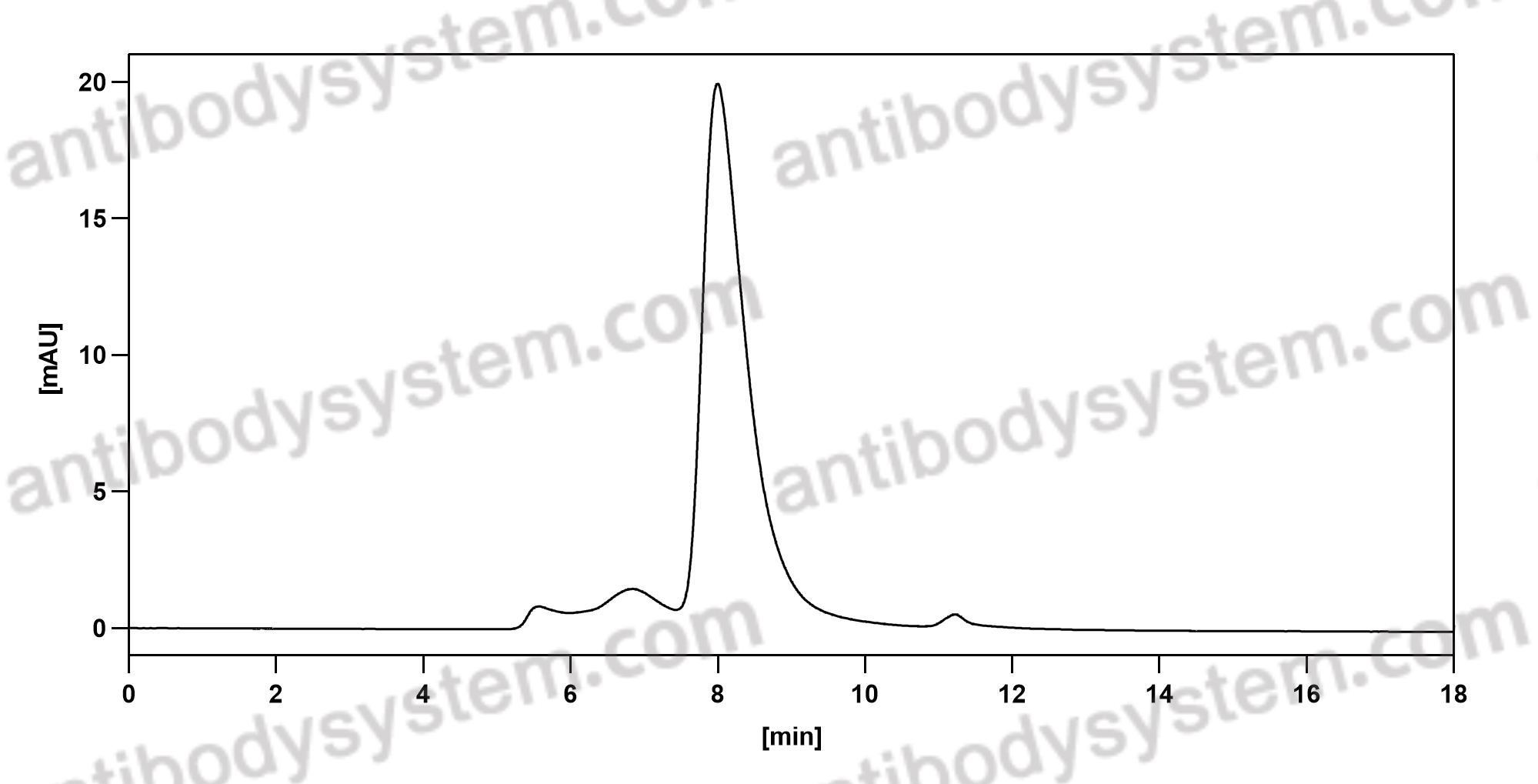
Galcanezumab in chronic migraine: The randomized, double-blind, placebo-controlled REGAIN study, PMID: 30446596
Galcanezumab in migraine prevention: a systematic review and meta-analysis of randomized controlled trials, PMID: 32426040
Galcanezumab for migraine, PMID: 32921891
Evaluation of Galcanezumab for the Prevention of Episodic Migraine: The EVOLVE-1 Randomized Clinical Trial, PMID: 29813147
Galcanezumab: First Global Approval, PMID: 30378008
Trial of Galcanezumab in Prevention of Episodic Cluster Headache, PMID: 31291515
Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE-2 Phase 3 randomized controlled clinical trial, PMID: 29848108
Efficacy and Safety of Galcanezumab for the Preventive Treatment of Migraine: A Narrative Review, PMID: 32319039
Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial, PMID: 32949542
Galcanezumab for the prevention of cluster headache, PMID: 32702245
Galcanezumab, PMID: 31643470
An Evidence-Based Review of Galcanezumab for the Treatment of Migraine, PMID: 33010021
Preventive effects of galcanezumab in adult patients with episodic or chronic migraine are persistent: data from the phase 3, randomized, double-blind, placebo-controlled EVOLVE-1, EVOLVE-2, and REGAIN studies, PMID: 30594122
Galcanezumab: A Review in the Prevention of Migraine and Treatment of Episodic Cluster Headache, PMID: 32504377
Galcanezumab, PMID: 30372007
A phase 3, long-term, open-label safety study of Galcanezumab in patients with migraine, PMID: 30413151
Galcanezumab for the prevention of migraine, PMID: 33291980
Reducing Episodic Cluster Headaches: Focus on Galcanezumab, PMID: 32753938
Erenumab and galcanezumab in chronic migraine prevention: effects after treatment termination, PMID: 31159727
The treatment efficacy of galcanezumab for migraine: A meta-analysis of randomized controlled trials, PMID: 31581028
Effect of Different Doses of Galcanezumab vs Placebo for Episodic Migraine Prevention: A Randomized Clinical Trial, PMID: 29255900
Galcanezumab CONQUERs migraine prevention, PMID: 32949530
Efficacy and safety of galcanezumab for preventive treatment of migraine: a systematic review and meta-analysis, PMID: 32006159
Fremanezumab (Ajovy) and galcanezumab (Emgality) for migraine prevention, PMID: 30681655
Migraine overview and summary of current and emerging treatment options, PMID: 30681821
Once-monthly galcanezumab for the prevention of migraine in adults: an evidence-based descriptive review and potential place in therapy, PMID: 31043785
Galcanezumab for the Management of Migraine: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials, PMID: 33376635
Medication overuse in a subgroup analysis of phase 3 placebo-controlled studies of galcanezumab in the prevention of episodic and chronic migraine, PMID: 33143451
Drug profile: galcanezumab for prevention of cluster headache, PMID: 33206562
Galcanezumab (Emgality) for Migraine and Cluster Headaches, PMID: 32293845
Safety profile of erenumab, galcanezumab and fremanezumab in pregnancy and lactation: Analysis of the WHO pharmacovigilance database, PMID: 33435709
Galcanezumab: a humanized monoclonal antibody for the prevention of migraine and cluster headache, PMID: 32055802
Safety of galcanezumab in patients with episodic migraine: A randomized placebo-controlled dose-ranging Phase 2b study, PMID: 29310444
Assessment of immunogenicity from galcanezumab phase 3 trials in patients with episodic or chronic migraine, PMID: 32340471
Efficacy of galcanezumab in patients with chronic migraine and a history of preventive treatment failure, PMID: 31104507
Safety and tolerability of monthly galcanezumab injections in patients with migraine: integrated results from migraine clinical studies, PMID: 31952501
A new era for migraine: Pharmacokinetic and pharmacodynamic insights into monoclonal antibodies with a focus on galcanezumab, an anti-CGRP antibody, PMID: 30917684
Safety and efficacy of galcanezumab in Taiwanese patients: a post-hoc analysis of phase 3 studies in episodic and chronic migraine, PMID: 32845740
Population Pharmacokinetics of Galcanezumab, an Anti-CGRP Antibody, Following Subcutaneous Dosing to Healthy Individuals and Patients With Migraine, PMID: 31482569
Two randomized migraine studies of galcanezumab: Effects on patient functioning and disability, PMID: 31270220
Monoclonal antibodies for the prevention of migraine, PMID: 31550937
Effect of Galcanezumab Following Treatment Cessation in Patients With Migraine: Results From 2 Randomized Phase 3 Trials, PMID: 30942898
Galcanezumab in episodic migraine: subgroup analyses of efficacy by high versus low frequency of migraine headaches in phase 3 studies (EVOLVE-1 & EVOLVE-2), PMID: 31253091
Impact of galcanezumab on total pain burden: findings from phase 3 randomized, double-blind, placebo-controlled studies in patients with episodic or chronic migraine (EVOLVE-1, EVOLVE-2, and REGAIN trials), PMID: 33069214
CGRP as the target of new migraine therapies - successful translation from bench to clinic, PMID: 29691490
Positive response to galcanezumab following treatment failure to onabotulinumtoxinA in patients with migraine: post hoc analyses of three randomized double-blind studies, PMID: 31595600
CGRP Antibodies as Prophylaxis in Migraine, PMID: 30550780
Evaluation of injection-site-related adverse events with galcanezumab: a post hoc analysis of phase 3 studies in participants with migraine, PMID: 32429851
Role of CGRP in Migraine, PMID: 30725283
What's New in the Treatment of Migraine?, PMID: 31393282
Retrospective cohort study of anti-CGRP monoclonal antibody unresponsive migraine individuals treated with atogepant: The RESCUE study., PMID:40501126
Management of local and delayed cutaneous hypersensitivity reactions to anti-CGRP antibodies: implications for continuing treatment., PMID:40456979
Repurposed versus disease-specific medicinals for the prophylaxis of migraine: an updated systematic review., PMID:40447296
Postmarketing safety of migraine prophylactic monoclonal antibodies: An EudraVigilance database analysis of eptinezumab, fremanezumab, galcanezumab, and erenumab., PMID:40439253
Usefulness of the Support Video "Talking Picture Book" for Overcoming Hesitancy to Start Galcanezumab Therapy., PMID:40350750
Calcitonin gene-related peptide-targeted therapies for migraine., PMID:40343132
Genetic migraine disorders and the response to calcitonin gene-related peptide antagonist treatment., PMID:40341526
Efficacy of monoclonal antibodies against CGRP in migraine patients with fibromyalgia comorbidity: a retrospective monocentric observational study., PMID:40329175
Increased infection risk in patients on preventive CGRP-targeting therapies- a meta-analysis and clinical effect assessment., PMID:40281424
Identifying signals of disproportionate reporting for calcitonin gene-related peptide inhibitors: real-world evidence from the FDA adverse event reporting system., PMID:40261259
Migraine treatment with CGRP monoclonal antibodies: patient evaluation of a mandatory treatment holiday in Belgium., PMID:40210844
Monthly Headaches and Severity in Patients on Galcanezumab or Traditional Preventive Migraine Medication: A 24-Month Claims and Electronic Health Records Study., PMID:40210791
Predictors of Response to Treatment With Anti-calcitonin Gene-Related Peptide (CGRP) Antibodies in Real-World Patients With Episodic Migraine: A Two- and Four-Month Prospective Study., PMID:40206906
[Successful Management of Wearing-off effect with Eptinezumab: Lessons from a case with Chronic Migraine Refractory to Two Subcutaneous CGRP Antibodies]., PMID:40191903
A National Cross-Sectional Survey on Real-World Experiences of Calcitonin Gene-Related Peptide (CGRP) Monoclonal Antibody Use in Adults with Migraine in Finland., PMID:40189729
Health care resource utilization and direct costs incurred over 12 months by patients with migraine initiating self-injectable calcitonin gene-related peptide monoclonal antibodies: A US real-world study., PMID:40152794
Tracking Migraine Symptoms: A Longitudinal Comparison of Smartphone-Based Headache Diaries and Clinical Interviews., PMID:40137454
Patient satisfaction with calcitonin gene-related peptide monoclonal antibodies in migraine: A multicenter prospective cohort study., PMID:40135519
CROATIAN GUIDELINES FOR SPECIFIC PREVENTIVE TREATMENT OF MIGRAINE WITH MONOCLONAL ANTIBODIES TARGETING CALCITONIN GENE-RELATED PEPTIDE (CGRP) (EPTINEZUMAB, FREMANEZUMAB, AND GALCANEZUMAB) OR THE CGRP RECEPTOR (ERENUMAB)., PMID:40104234
Efficacy and continuability of 675 mg fremanezumab administration over 2 years., PMID:40069630
Treatment Patterns and Healthcare Resource Utilization by Gender and Migraine Frequency in Adult Patients Receiving Galcanezumab Versus Standard of Care Preventive Medications Over 24 months: A United States Retrospective Claims Study., PMID:40046564
Real-World Lessons with Fremanezumab as the Third Available CGRP Monoclonal Antibody in a Third-Level Hospital: Focus on the Factors Predicting Response., PMID:40004586
Review: An Update on CGRP Monoclonal Antibodies for the Preventive Treatment of Episodic Migraine., PMID:39998706
Peripherally acting anti-CGRP monoclonal antibodies attenuate cortical resting-state connectivity in migraine patients., PMID:39995155
Health Technology Assessment: Evaluation of 8 CGRP-Targeted Therapy Drugs for the Treatment of Migraine., PMID:39991088
The effect of calcitonin gene-related peptide monoclonal antibodies on restless legs syndrome in patients with migraine., PMID:39972460
Disproportionality analysis of Raynaud's phenomenon associated with calcitonin gene-related peptide inhibitors using the Food and Drug Administration adverse event reporting system., PMID:39955348
Shift from chronic to episodic migraine frequency in a long-term phase 3 study of galcanezumab., PMID:39901101
Prevention of Episodic Migraine Headache Using Pharmacologic Treatments in Outpatient Settings: A Clinical Guideline From the American College of Physicians., PMID:39899861
Switching between anti-CGRP monoclonal antibodies in migraine prophylaxis., PMID:39884968
Pharmacological differences and switching among anti-CGRP monoclonal antibodies: A narrative review., PMID:39825578
Medication underuse in real-life practice: the impact of galcanezumab towards achieving very low frequency episodic migraine in a southeast Asian middle-income nation., PMID:39825238
Efficacy and Safety of Switching Between Anti-CGRP Monoclonal Antibodies: A Detailed Monthly and Long-term Follow-up Study and Literature Review., PMID:39756881
Vertebral artery dissection in a patient with migraine treated with calcitonin gene-related peptide monoclonal antibody: a case report and FAERS database analysis., PMID:39748278
Simultaneous Immediate and Delayed Hypersensitivity to Galcanezumab-Case Report., PMID:39739564
Interictal burden in migraine patients at the outset of CGRP monoclonal antibody prevention., PMID:39695402
Efficacy and safety of galcanezumab for cluster headache preventive treatment: a systematic review and meta-analysis., PMID:39686845
Healthcare Utilization, Costs, and Treatment Discontinuation in Adults with Episodic Migraine Initiating Galcanezumab Versus Rimegepant: A US Retrospective Claims Analysis., PMID:39680312
Comparison of Early-onset Efficacy of Anti-calcitonin Gene-related Peptide Monoclonal Antibodies for Patients with Migraine in Real-world Clinical Practice: Study Protocol for an Exploratory Clinical Trial., PMID:39662907
World neurology updates: Other primary headache disorder - Treatment., PMID:39654689
Real-world experience of galcanezumab in the prevention of migraine in Spain: a systematic literature review., PMID:39639987
Cluster headache and galcanezumab: the first real-world Brazilian study and an expert consensus on its use among other treatments., PMID:39627689
One-step ultra-rapid immunoassay of calcitonin gene-related peptide for migraine diagnosis., PMID:39608279
Anti-Calcitonin Gene-Related Peptide Monoclonal Antibody Is Effective for Preventing Migraine Aura Without Headache., PMID:39585056
Galcanezumab add-on in refractory cluster headache. A case series., PMID:39558673
Benefit-risk assessment based on number needed to treat and number needed to harm: Atogepant vs. calcitonin gene-related peptide monoclonal antibodies., PMID:39558612
Patient-reported outcomes related to migraine burden among patients treated with standard-of-care preventive medications or calcitonin gene-related monoclonal antibodies: a United States and Europe cross-sectional survey., PMID:39523857
Adding corticosteroids to galcanezumab in medication overuse headache: A three-arm head-to-head prospective observational cohort study., PMID:39510936
Efficacy of eptinezumab in non-responders to subcutaneous monoclonal antibodies against CGRP and the CGRP receptor: A retrospective cohort study., PMID:39469839
Hypersensitivity to the CGRP Inhibitor Monoclonal Antibodies Galcanezumab, Erenumab, and Fremanezumab With Tolerance to Eptinezumab., PMID:39441051