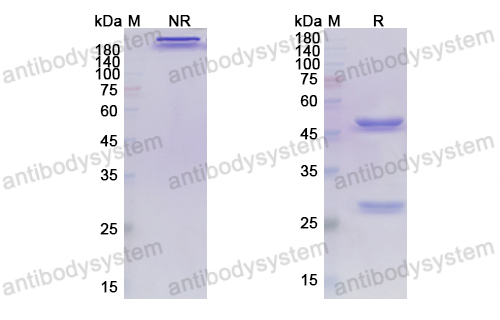
Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura, PMID: 30625070
Caplacizumab: First Global Approval, PMID: 30298461
Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura, PMID: 26863353
Caplacizumab in adult patients with acquired thrombotic thrombocytopenic purpura, PMID: 32095224
Caplacizumab: an anti-von Willebrand factor antibody for the treatment of thrombotic thrombocytopenic purpura, PMID: 32588878
Caplacizumab: a change in the paradigm of thrombotic thrombocytopenic purpura treatment, PMID: 31359806
A regimen with caplacizumab, immunosuppression, and plasma exchange prevents unfavorable outcomes in immune-mediated TTP, PMID: 33150928
Caplacizumab to treat immune-mediated thrombotic thrombocytopenic purpura, PMID: 31250841
Cost effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura, PMID: 33280030
Real-world experience with caplacizumab in the management of acute TTP, PMID: 33150355
Real-world data confirm the effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura, PMID: 32634236
Efficacy and safety of open-label caplacizumab in patients with exacerbations of acquired thrombotic thrombocytopenic purpura in the HERCULES study, PMID: 31691462
Caplacizumab, PMID: 30933455
ADAMTS13 and VWF activities guide individualized caplacizumab treatment in patients with aTTP, PMID: 32634237
Clinical pharmacology of caplacizumab for the treatment of patients with acquired thrombotic thrombocytopenic purpura, PMID: 30977686
A critical evaluation of caplacizumab for the treatment of acquired thrombotic thrombocytopenic purpura, PMID: 32876503
Should all patients with immune-mediated thrombotic thrombocytopenic purpura receive caplacizumab?, PMID: 33236389
Caplacizumab (Cablivi) for iTTP, PMID: 33429409
Caplacizumab as an emerging treatment option for acquired thrombotic thrombocytopenic purpura, PMID: 31118566
Caplacizumab: frequent local skin reactions, PMID: 32997190
Caplacizumab for relapsing thrombotic thrombocytopenic purpura, PMID: 31177334
Thrombotic thrombocytopenic purpura, PMID: 28416507
Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura, PMID: 31042846
Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura, PMID: 31042845
Caplacizumab for thrombotic thrombocytopenic purpura, PMID: 33664548
The Therapeutic Potential of Nanobodies, PMID: 31686399
Caplacizumab, PMID: 34251781
Caplacizumab for treatment of thrombotic thrombocytopenic purpura in a patient with anaphylaxis to fresh-frozen plasma, PMID: 32358818
Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura. Reply, PMID: 31042847
Clinical Efficacy and Safety Profile of Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura, PMID: 31576256
Caplacizumab treatment for acquired refractory thrombotic thrombocytopenic purpura, PMID: 32738052
Refractory Auto-Immune Thrombotic Thrombocytopenic Pupura Successfully Treated With Caplacizumab, PMID: 33195299
Role of caplacizumab in the treatment of acquired thrombotic thrombocytopenic purpura, PMID: 32605495
ISTH guidelines for treatment of thrombotic thrombocytopenic purpura, PMID: 32914526
Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura (HERCULES trial), PMID: 31318128
Pharmacoeconomic Review Report: Caplacizumab (Cablivi): (Sanofi Genzyme, a division of Sanofi-Aventis Canada Inc.) [Internet], PMID: 33570888
New Therapeutic Targets and Treatment Options for Thrombotic Microangiopathy: Caplacizumab and Ravulizumab, PMID: 32715723
Counting the cost of caplacizumab, PMID: 33599760
Antibodies to watch in 2019, PMID: 30516432
Caplacizumab Therapy without Plasma Exchange for Acquired Thrombotic Thrombocytopenic Purpura, PMID: 31269374
Correction to: Caplacizumab: First Global Approval, PMID: 30511322
Successful use of caplacizumab in a case of refractory acquired thrombotic thrombocytopenic purpura following subacute thyroiditis, PMID: 33223471
ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura, PMID: 32914582
Effective and safe off-label use of caplacizumab treatment in a middle-aged obese male, PMID: 33285299
Clinical Review Report: Caplacizumab (Cablivi): (Sanofi Genzyme, a division of Sanofi-Aventis Canada Inc.) [Internet], PMID: 33600100
Caplacizumab reduces the frequency of major thromboembolic events, exacerbations and death in patients with acquired thrombotic thrombocytopenic purpura, PMID: 28445600
Shared decision making, thrombotic thrombocytopenic purpura, and caplacizumab, PMID: 31894592
Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura, PMID: 27332911
Nanobody Engineering: Toward Next Generation Immunotherapies and Immunoimaging of Cancer, PMID: 31544819
Caplacizumab for Acute Thrombotic Thrombocytopenic Purpura, PMID: 34109052
Relapse and beyond: Navigating the long-term clinical impacts of immune thrombotic thrombocytopenic purpura., PMID:40505364
Thrombotic Thrombocytopenic Purpura Without Neurological Involvement: A Case Report and Review of the Diagnostic and Treatment Strategies., PMID:40486424
The TMA team and TTP pathway improved outcomes in a cohort with Thrombotic thrombocytopenic purpura., PMID:40478853
Resistant thrombotic thrombocytopenic purpura successfully treated with caplacizumab preceding acute myeloid leukemia., PMID:40444399
Immune Thrombotic Thrombocytopenic Purpura: A Review., PMID:40388146
Early caplacizumab and obinutuzumab enable successful treatment of relapsing thrombotic thrombocytopenic purpura without therapeutic plasma exchange: a case report., PMID:40356910
Cost-Effectiveness and Value of Information Analyses of Caplacizumab for the Treatment of Thrombotic Thrombocytopenic Purpura in Brazil., PMID:40272651
Rapid ADAMTS13 activity assays for thrombotic thrombocytopenic purpura: a systematic review and meta-analysis., PMID:40258188
Caplacizumab treatment in elderly patients with iTTP: Experience from the Spanish TTP Registry., PMID:40242663
Caplacizumab use in immune-mediated thrombotic thrombocytopenic purpura: an international multicentre retrospective Cohort study (The Capla 1000+ project)., PMID:40235949
Optimization of nanobody caplacizumab via computational design., PMID:40226960
Caplacizumab in acute thrombotic throbocytopenic purpura refractory to standard treatment., PMID:40210561
A Descriptive 5-Year Analysis of the Demographics and Therapies for Patients With Immune Thrombotic Thrombocytopenic Purpura in the USA: A Multicenter Study of 390 Disease Episodes From 2017 to 2021., PMID:40145682
Challenges in managing iTTP: insights into ADAMTS13 inhibitor boosting during caplacizumab therapy., PMID:40105947
Delayed Recovery of a Disintegrin-Like and Metalloproteinase With Thrombospondin Type 1 Motif 13 (ADAMTS13) Activity in Immune Thrombotic Thrombocytopenic Purpura Treated With Caplacizumab and Rituximab., PMID:40091942
Early administration of caplacizumab combined with plasma exchange for thrombotic microangiopathy due to malignant hypertension: a case report., PMID:40029564
Cost-effectiveness of caplacizumab in immune thrombotic thrombocytopenic purpura in the United States., PMID:40021467
Immune-mediated TTP secondary to checkpoint inhibitor use in a patient with stage IV melanoma., PMID:40000048
Preventative and therapeutic effects of a novel humanized anti-glycoprotein Ibα fragment of antigen-binding region in a murine model of thrombotic thrombocytopenic purpura., PMID:39956431
Research trends in the use of nanobodies for cancer therapy., PMID:39922288
Impact of new medications on the treatment of immune TTP., PMID:39912777
Characterization of bleeding in thrombotic thrombocytopenic purpura in the precaplacizumab era: a retrospective nationwide analysis., PMID:39830971
A Rare But Fatal Toxicity: Immune Checkpoint Inhibitor-Related Acquired Thrombotic Thrombocytopenic Purpura., PMID:39811422
GC1126A, a novel ADAMTS13 mutein, evades autoantibodies in immune-mediated thrombotic thrombocytopenic purpura., PMID:39794345
Thrombotic thrombocytopenic purpura with juvenile systemic lupus erythematosus: successful treatment with caplacizumab and rituximab., PMID:39696445
Complex immunogenicity assessment in caplacizumab-treated patients with immune-mediated thrombotic thrombocytopenic purpura who have received plasma exchange., PMID:39687921
Coronavirus Disease 2019-Associated Thrombotic Microangiopathy: A Single-Center Experience., PMID:39596538
Bortezomib for rituximab-refractory immune-mediated thrombotic thrombocytopenic purpura in the caplacizumab era: an Italian multicenter study., PMID:39549837
A high-performance protein preparation approach in a single column-free step., PMID:39537535
Systematic Review of Individual Patient Data COVID-19 Infection and Vaccination-Associated Thrombotic Microangiopathy., PMID:39534187
Real-World Data on Effectiveness and Safety of First-Line Use of Caplacizumab in Italian Centers for the Treatment of Thrombotic Thrombocytopenic Purpura: The Roscapli Study., PMID:39518700
[100 years thrombotic thrombocytopenic purpura (TTP) - lessons learned?]., PMID:39504978
Discovery of nanobodies: a comprehensive review of their applications and potential over the past five years., PMID:39455963
Taylor A, Keogh L, Dickens E, et al. Caplacizumab in pediatric immune thrombotic thrombocytopenic purpura: the UK TTP Registry experience. Blood Adv. 2024;8(17):4563-4567., PMID:39453632
[Not Available]., PMID:39442507
Vitamin B12 Deficiency in Thrombotic Thrombocytopenic Purpura-Like Cases., PMID:39372153
A Rare Intersection: Managing Thrombotic Thrombocytopenic Purpura in the Context of Dengue Fever., PMID:39371874
Refining the standard of care in immune thrombotic thrombocytopenic purpura., PMID:39356816
An exploration into CTEPH medications: Combining natural language processing, embedding learning, in vitro models, and real-world evidence for drug repurposing., PMID:39264975
Initial US tertiary health care system experience using caplacizumab in patients with immune thrombotic thrombocytopenic purpura., PMID:39259327
Caplacizumab as an add-on therapy in a 7-year-old girl with exacerbated immune-mediated thrombotic thrombocytopenic purpura, a case report and literature review., PMID:39233868
Caplacizumab improves clinical outcomes and is well tolerated across clinically relevant subgroups of patients with immune-mediated thrombotic thrombocytopenic purpura., PMID:39221451
Clinical, biological, prognostic characteristics of patients with immune-mediated thrombotic thrombocytopenic purpura and Sjögren's disease., PMID:39209728
TTP and pregnancy., PMID:39197429
Iatrogenic hemorrhage and extensive venous thromboembolism during iTTP treatment with caplacizumab-A case report., PMID:39157617
ADAMTS13 in the New Era of TTP., PMID:39125707
Immune thrombotic thrombocytopenic purpura: pathogenesis and novel therapies: a narrative review., PMID:39100389
Frontline use of rituximab may prevent ADAMTS13 inhibitor boosting during caplacizumab treatment in patients with iTTP: post hoc analysis of a phase 2/3 study in Japan., PMID:39095866
The history of thrombotic thrombocytopenic purpura research: a narrative review., PMID:39049905