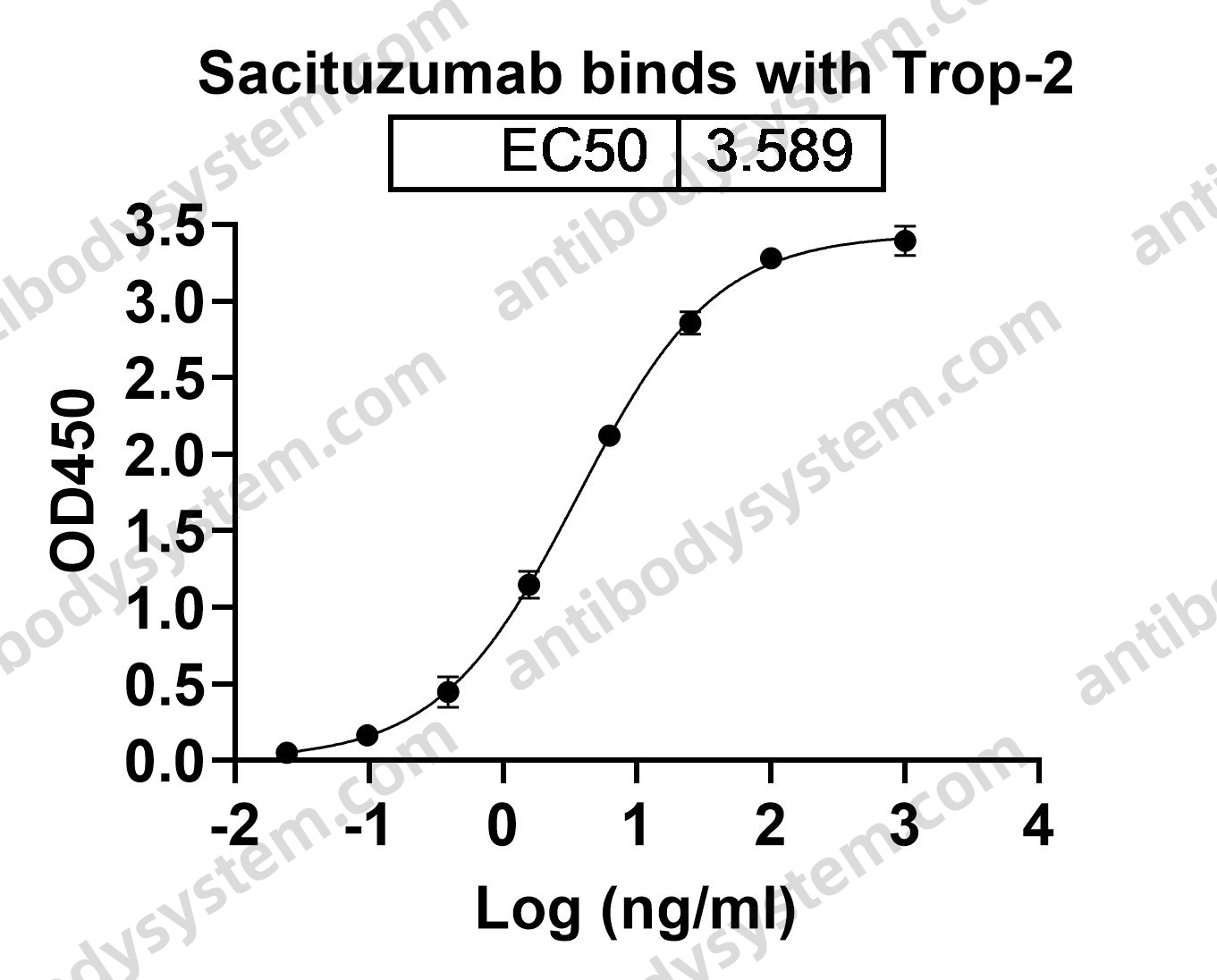
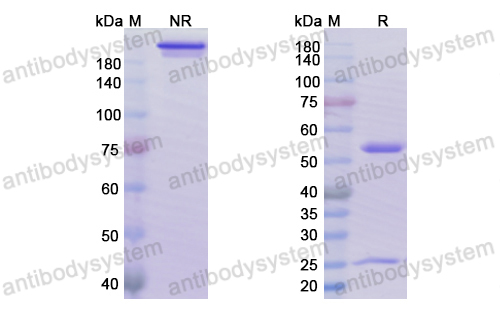
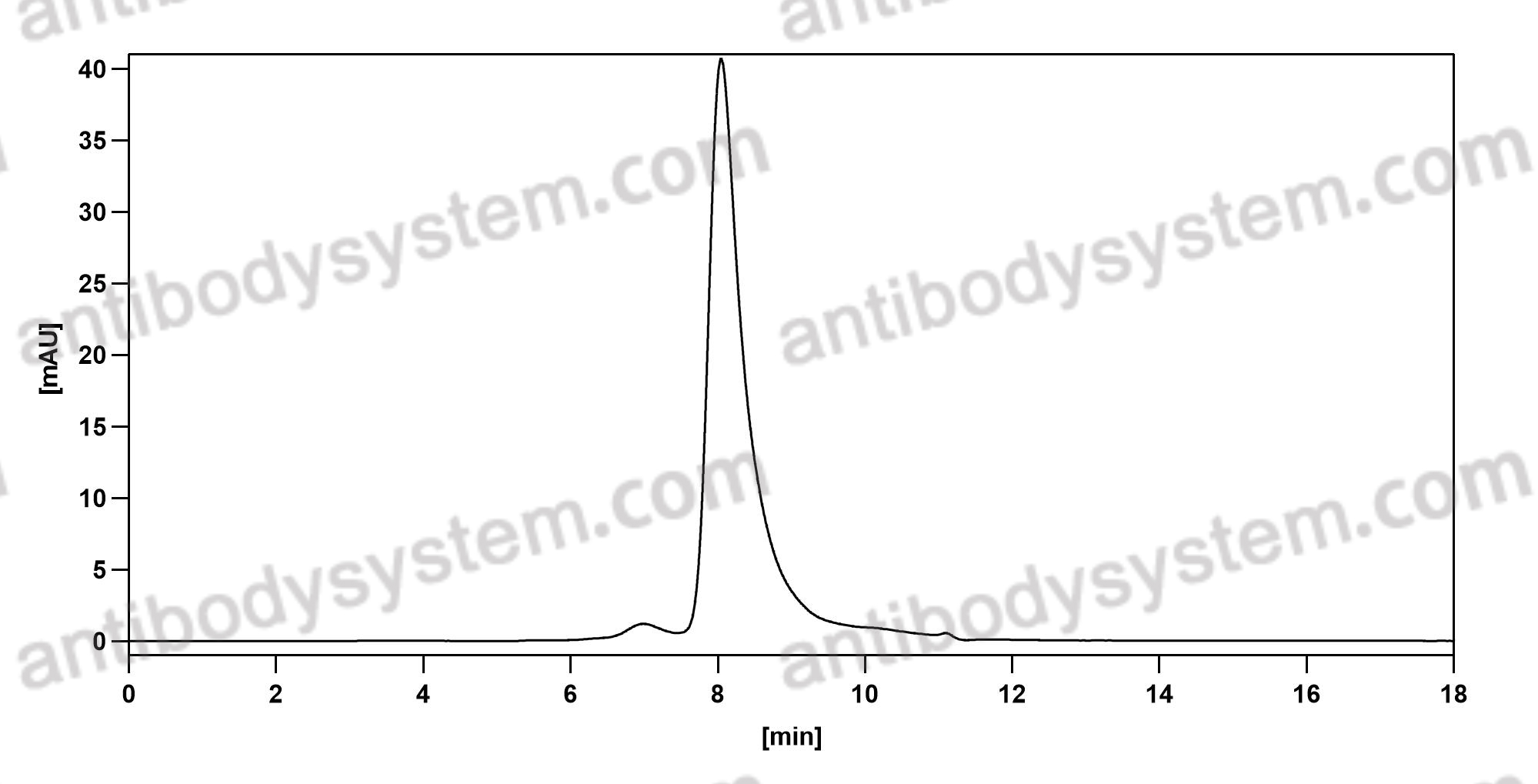
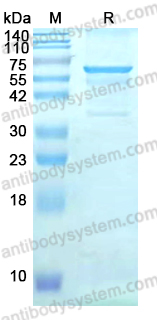
Sacituzumab Govitecan: First Approval, PMID: 32529410
Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer, PMID: 30786188
Sacituzumab govitecan: breakthrough targeted therapy for triple-negative breast cancer, PMID: 31398063
Sacituzumab govitecan: antibody-drug conjugate in triple-negative breast cancer and other solid tumors, PMID: 31584574
Sacituzumab govitecan: an antibody-drug conjugate, PMID: 28503956
Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy, PMID: 32301634
Sacituzumab Govitecan-hziy: An Antibody-Drug Conjugate for the Treatment of Refractory, Metastatic, Triple-Negative Breast Cancer, PMID: 33070624
Antibody-drug conjugates targeting TROP-2 and incorporating SN-38: A case study of anti-TROP-2 sacituzumab govitecan, PMID: 31208270
Efficacy and Safety of Anti-Trop-2 Antibody Drug Conjugate Sacituzumab Govitecan (IMMU-132) in Heavily Pretreated Patients With Metastatic Triple-Negative Breast Cancer, PMID: 28291390
FDA Approval Summary: Accelerated Approval of Sacituzumab Govitecan-hziy for Third-line Treatment of Metastatic Triple-negative Breast Cancer, PMID: 33168656
Sacituzumab govitecan in previously treated hormone receptor-positive/HER2-negative metastatic breast cancer: final results from a phase I/II, single-arm, basket trial, PMID: 32946924
TROPiCS-02: A Phase III study investigating sacituzumab govitecan in the treatment of HR+/HER2- metastatic breast cancer, PMID: 32223649
First-in-Human Trial of a Novel Anti-Trop-2 Antibody-SN-38 Conjugate, Sacituzumab Govitecan, for the Treatment of Diverse Metastatic Solid Tumors, PMID: 25944802
Sacituzumab Govitecan (IMMU-132), an Anti-Trop-2/SN-38 Antibody-Drug Conjugate: Characterization and Efficacy in Pancreatic, Gastric, and Other Cancers, PMID: 25915780
Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: Safety and pharmacokinetics, PMID: 28558150
Sacituzumab for the treatment of triple-negative breast cancer: the poster child of future therapy?, PMID: 30507322
Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC), PMID: 26101915
Predictive biomarkers for sacituzumab govitecan efficacy in Trop-2-expressing triple-negative breast cancer, PMID: 33196706
Antibodies to watch in 2020, PMID: 31847708
A closer look at sacituzumab govitecan-hziy, PMID: 33406063
Therapy of Small Cell Lung Cancer (SCLC) with a Topoisomerase-I-inhibiting Antibody-Drug Conjugate (ADC) Targeting Trop-2, Sacituzumab Govitecan, PMID: 28679770
Therapy of Advanced Non-Small-Cell Lung Cancer With an SN-38-Anti-Trop-2 Drug Conjugate, Sacituzumab Govitecan, PMID: 28548889
Antibody-drug conjugates in metastatic triple negative breast cancer: a spotlight on sacituzumab govitecan, ladiratuzumab vedotin, and trastuzumab deruxtecan, PMID: 33089726
Sacituzumab govitecan-hziy for triple-negative breast cancer, PMID: 30827748
Sacituzumab Govitecan-hziy in Triple-Negative Breast Cancer, PMID: 31189048
Enhanced Delivery of SN-38 to Human Tumor Xenografts with an Anti-Trop-2-SN-38 Antibody Conjugate (Sacituzumab Govitecan), PMID: 26106073
Sacituzumab Govitecan-hziy in Triple-Negative Breast Cancer. Reply, PMID: 31189049
Antibody-drug conjugates of 7-ethyl-10-hydroxycamptothecin: Sacituzumab govitecan and labetuzumab govitecan, PMID: 30822636
Sacituzumab govitecan: a promising antibody-drug conjugate for the treatment of poorly differentiated endometrial cancer, PMID: 33195736
Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer, PMID: 33882206
Trop2: Jack of All Trades, Master of None, PMID: 33187148
Preclinical activity of sacituzumab govitecan (IMMU-132) in uterine and ovarian carcinosarcomas, PMID: 32082489
Novel antibody-drug conjugates for triple negative breast cancer, PMID: 32426047
Antibodies to watch in 2019, PMID: 30516432
The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target, PMID: 29989029
Irinotecan: 25 years of cancer treatment, PMID: 31415916
Sacituzumab govitecan, an antibody-drug conjugate targeting trophoblast cell-surface antigen 2, shows cytotoxic activity against poorly differentiated endometrial adenocarcinomas in vitro and in vivo, PMID: 31891442
Cervical carcinomas that overexpress human trophoblast cell-surface marker (Trop-2) are highly sensitive to the antibody-drug conjugate sacituzumab govitecan, PMID: 31969666
Sacituzumab govitecan (Trodelvy) for metastatic triple-negative breast cancer, PMID: 33757114
Sacituzumab Govitecan (IMMU-132) in treatment-resistant uterine serous carcinoma: A case report, PMID: 29977989
The emerging role of antibody-drug conjugates in urothelial carcinoma, PMID: 32552213
Antibody-Drug Conjugates in Breast Cancer: a Comprehensive Review, PMID: 30931493
Antibody-Drug Conjugates in Urothelial Carcinomas, PMID: 32008109
Sacituzumab govitecan activity in advanced breast cancer, PMID: 28343977
Preclinical Activity of Sacituzumab Govitecan, an Antibody-Drug Conjugate Targeting Trophoblast Cell-Surface Antigen 2 (Trop-2) Linked to the Active Metabolite of Irinotecan (SN-38), in Ovarian Cancer, PMID: 32117765
The evolving management of metastatic triple negative breast cancer, PMID: 32563561
Sacituzumab Govitecan in Metastatic Breast Cancer, PMID: 34260847
Sacituzumab govitecan and trastuzumab deruxtecan: two new antibody-drug conjugates in the breast cancer treatment landscape, PMID: 34225076
Sacituzumab Govitecan for Metastatic Triple-Negative Breast Cancer: Clinical Overview and Management of Potential Toxicities, PMID: 34176192
Major clinical research advances in gynecologic cancer in 2019, PMID: 32319232
Evolution of TROP2: Biological insights and clinical applications., PMID:40517574
Trop-2 expression as a biomarker of response to sacituzumab govitecan in patients with HER2-negative metastatic breast cancer: A pilot study., PMID:40516178
Advances in targeted therapy for triple-negative breast cancer: a review of key antigens and recent advances., PMID:40515614
The antibody-drug conjugate sacituzumab govitecan (IMMU-132) represents a potential novel therapeutic strategy in cholangiocarcinoma., PMID:40509933
A Meta-Analysis of Patient-Reported Outcomes of Sacituzumab Govitecan Versus Treatment of Physician's Choice in Previously Treated HR+/HER- mBC Using Two Phase 3 (TROPiCS-02 and EVER-132-002) Trials., PMID:40507364
Advancing Antibody-Drug Conjugates: Precision Oncology Approaches for Breast and Pancreatic Cancers., PMID:40507272
Efficacy and Safety of TROP-2-Targeting Antibody-Drug Conjugate Treatment in Previously Treated Patients with Advanced Non-Small Cell Lung Cancer: A Systematic Review and Pooled Analysis of Reconstructed Patient Data., PMID:40507234
Comparative pharmacological analysis of fam-trastuzumab deruxtecan-nxki and sacituzumab govitecan-hziy: Two recently developed chemotherapies in the crucial battle against breast cancer., PMID:40502326
Synergistic strategies: ADC-PARP inhibitor combinations in triple-negative breast cancer therapy., PMID:40494034
Results of a phase 1/2 study of sacituzumab tirumotecan in patients with unresectable locally advanced or metastatic solid tumors refractory to standard therapies., PMID:40481574
Effectiveness of sacituzumab govitecan and management of neutropenia in patients with metastatic triple-negative breast cancer treated in real-world settings in the United States., PMID:40479864
Real-world clinical outcomes of sacituzumab govitecan after prior exposure to enfortumab vedotin in patients with metastatic urothelial carcinoma., PMID:40479863
Sacituzumab tirumotecan versus docetaxel for previously treated EGFR-mutated advanced non-small cell lung cancer: multicentre, open label, randomised controlled trial., PMID:40473437
The association of high body mass index with the safety and efficacy of sacituzumab govitecan in patients with metastatic triple-negative breast cancer from the ASCENT study., PMID:40460679
Mechanisms of resistance to antibody-drug conjugates in cancers., PMID:40460510
New Treatment Approaches for Triple-Negative Breast Cancer., PMID:40460322
Evaluation of sacituzumab govitecan for advanced/metastatic non-small cell lung cancer., PMID:40458070
Avelumab plus sacituzumab govitecan versus avelumab monotherapy as first-line maintenance treatment in patients with advanced urothelial carcinoma: JAVELIN Bladder Medley interim analysis., PMID:40456670
Sequential Antibody-Drug Conjugate Therapy in Patients With Metastatic Breast Cancer Treated With Sacituzumab Govitecan and Trastuzumab Deruxtecan., PMID:40440568
Last Resort? Rationale for Comprehensive Molecular Analysis in Treatment-Refractory R/M HNSCC: A Case Report of Remarkable Response to Sacituzumab Govitecan Following Molecular and Functional Characterization., PMID:40427091
ADCs and TCE in SCLC Therapy: The Beginning of a New Era?, PMID:40422520
Cost and Cost-Effectiveness of Treating Human Epidermal Growth Factor Receptor 2-Low Metastatic Breast Cancer., PMID:40397834
A Multicenter Real-Life Evaluation of Safety and Effectiveness of the Antibody-Drug Conjugate Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer., PMID:40387297
Update Breast Cancer 2024 Part 3 - Patients with Advanced Stage Breast Cancer., PMID:40386501
Progress of antibody-drug conjugates in the treatment of locally advanced or metastatic urothelial carcinoma: opportunities and challenges., PMID:40377724
Plain language summary of the EVOKE-01 study of sacituzumab govitecan vs docetaxel in patients with non-small cell lung cancer., PMID:40371894
[Translated article] Influence of the UGT1A1 gene polymorphism on treatment with sacituzumab govitecan. Narrative review., PMID:40368667
Decoding Clinical Trials in Metastatic Breast Cancer: Practical Insights for Optimal Therapy Sequencing., PMID:40367401
Delphi consensus on the management of adverse events in patients with metastatic triple-negative breast cancer treated with sacituzumab govitecan., PMID:40366333
The Clinical Outcomes and Safety of Sacituzumab Govitecan in Heavily Pretreated Metastatic Triple-Negative and HR+/HER2- Breast Cancer: A Multicenter Observational Study from Turkey., PMID:40361516
TROP2 expression and therapeutic targeting in uterine carcinosarcoma., PMID:40344963
Navigating antibody‒drug conjugates (ADCs): from metastatic to early breast cancer treatment strategies., PMID:40304863
MMP1-induced NF-κB activation promotes epithelial-mesenchymal transition and sacituzumab govitecan resistance in hormone receptor-positive breast cancer., PMID:40287412
Sacituzumab tirumotecan improves OS in mTNBC., PMID:40269173
[Diffuse interstitial lung disease induced by antibody-drug conjugates]., PMID:40263022
Antibody-Based Therapeutics in Small Cell Lung Cancer: A Narrative Review., PMID:40260055
Evaluation of Nectin-4 and Trop-2: Implications for Patient Outcomes and Therapy in Penile Cancer., PMID:40252843
Targeting Lung Cancer with Precision: The ADC Therapeutic Revolution., PMID:40238068
Multicenter retrospective cohort study of the sequential use of the antibody-drug conjugates (ADCs) trastuzumab deruxtecan (T-DXd) and sacituzumab govitecan (SG) in patients with HER2-low metastatic breast cancer (MBC)., PMID:40234477
Sacituzumab tirumotecan in previously treated metastatic triple-negative breast cancer: a randomized phase 3 trial., PMID:40217078
Sacituzumab tirumotecan in advanced non-small-cell lung cancer with or without EGFR mutations: phase 1/2 and phase 2 trials., PMID:40210967
Antibody-drug conjugates in breast cancer treatment: resistance mechanisms and the role of therapeutic sequencing., PMID:40201309
Trop2-expression in primary vulvar squamous cell carcinoma., PMID:40184712
Safety and Tolerability of Concurrent Radiotherapy and Sacituzumab Govitecan in Metastatic Breast Cancer., PMID:40178882
Advances in adoptive cell therapies in small cell lung cancer., PMID:40160238
Toxicities and management strategies of emerging antibody-drug conjugates in breast cancer., PMID:40151551
Direct Preparation of Site-Specific Antibody-Drug Conjugates with Unpurified Antibodies in Culture Medium., PMID:40151029
Camptothein-Based Anti-Cancer Therapies and Strategies to Improve Their Therapeutic Index., PMID:40149365
Influence of the UGT1A1 gene polymorphism on treatment with sacituzumab govitecan. Narrative review., PMID:40140308
Q-TWiST Analysis of Sacituzumab Govitecan vs. Chemotherapy in Previously Treated Patients with HR+/HER2- Metastatic Breast Cancer., PMID:40136373