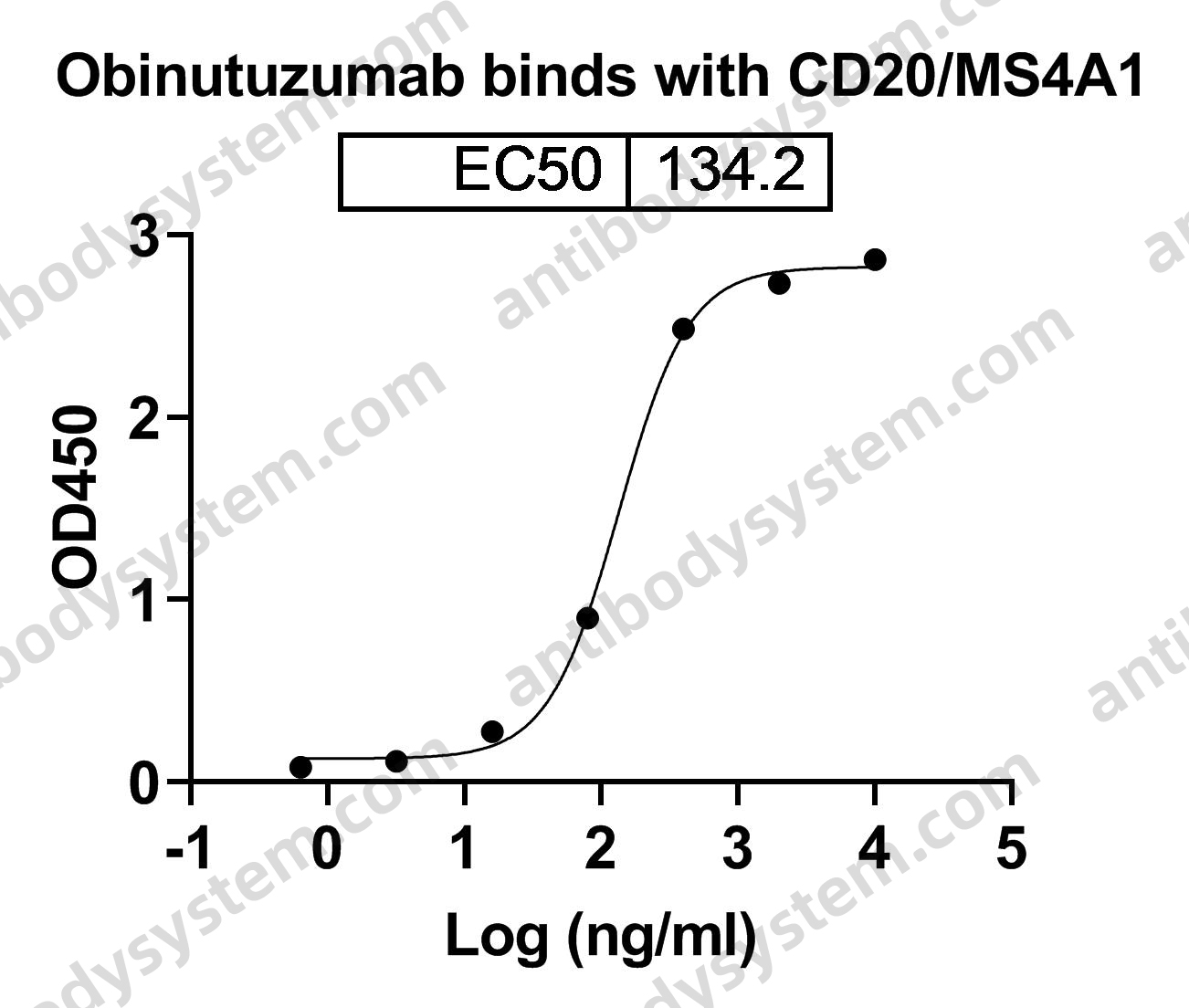
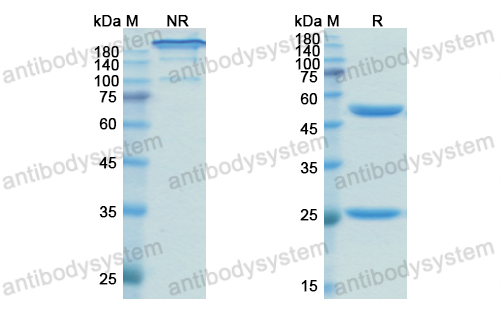
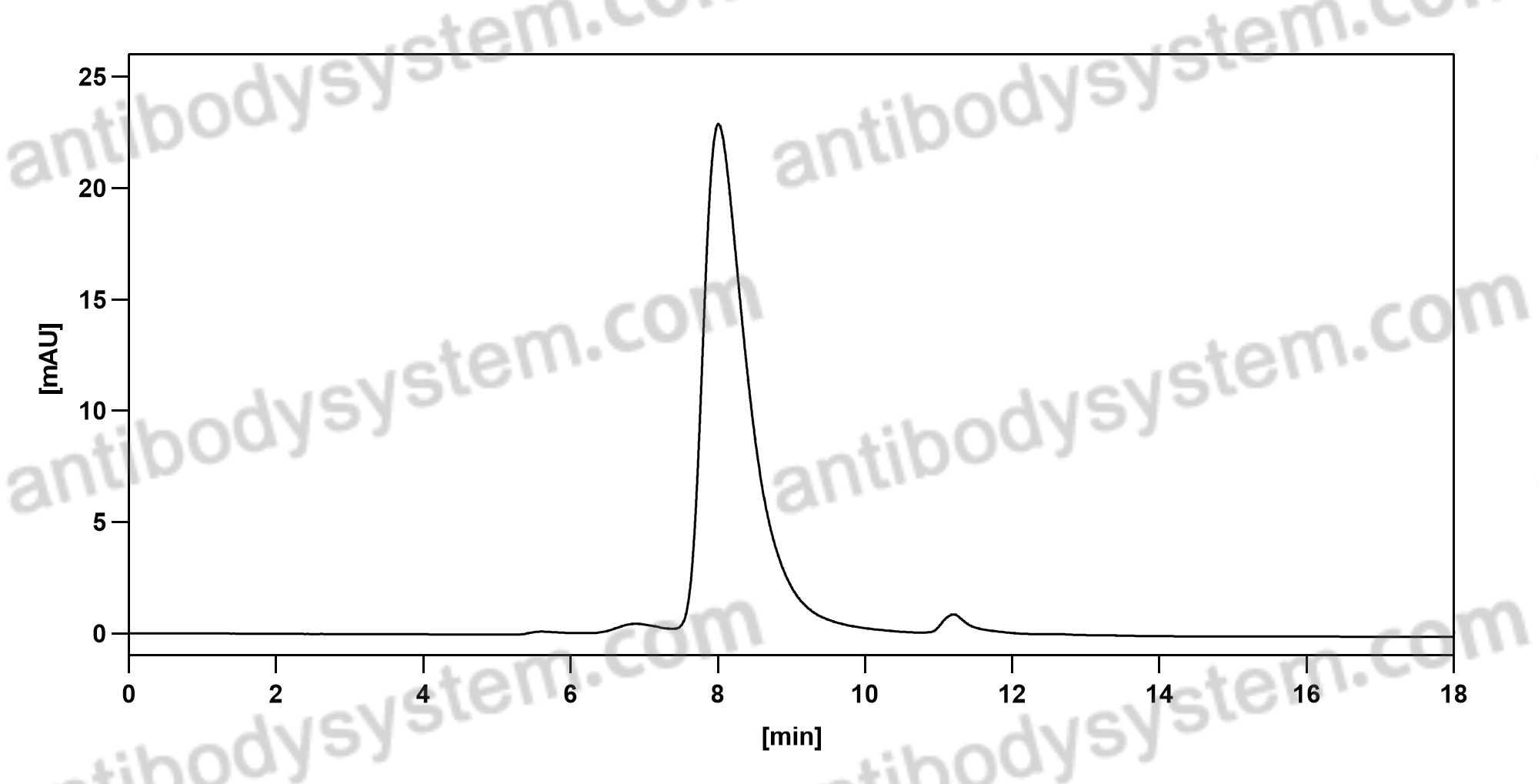
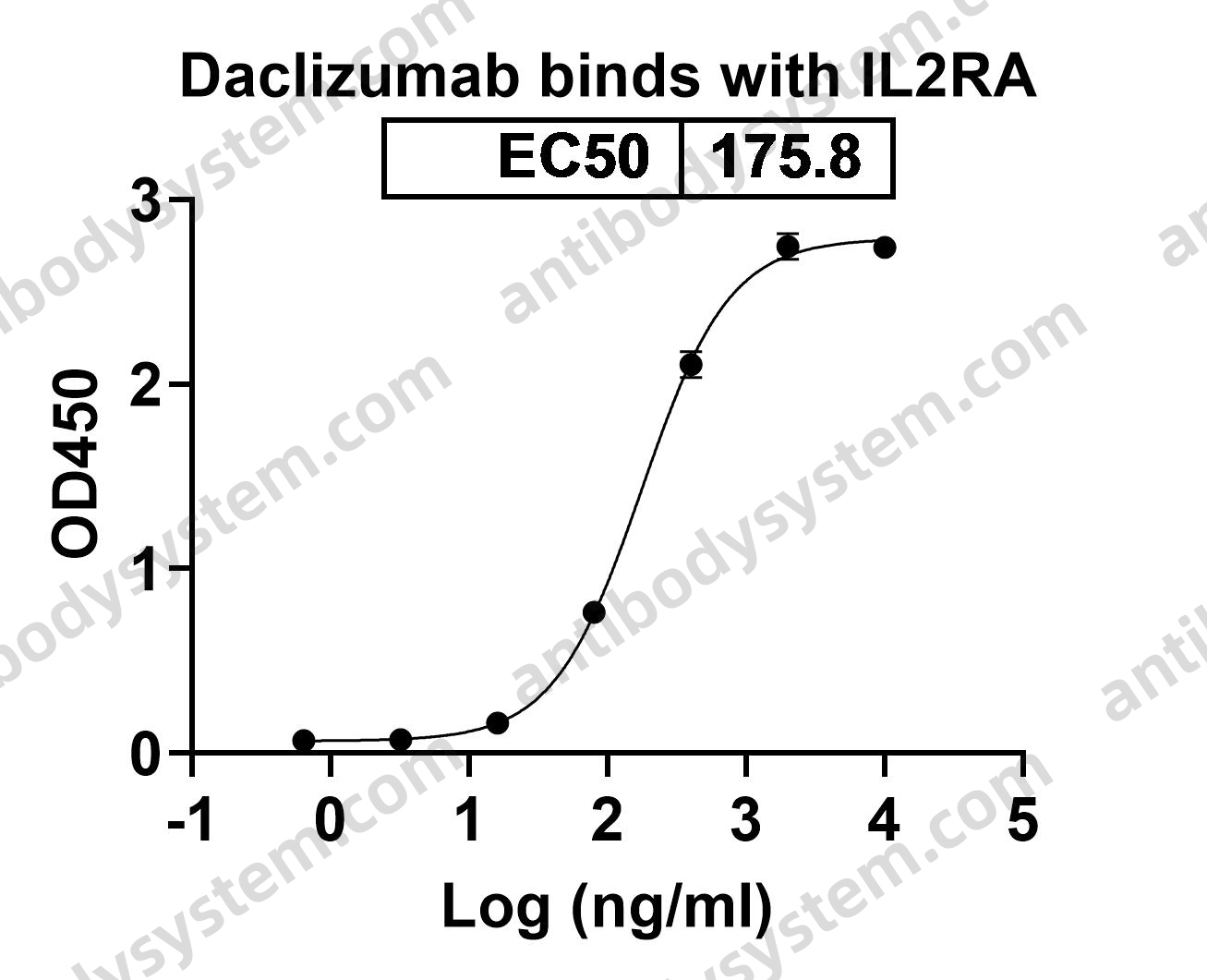
A tale of two antibodies: obinutuzumab versus rituximab, PMID: 29741753
Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions, PMID: 31166681
Obinutuzumab for the First-Line Treatment of Follicular Lymphoma, PMID: 28976863
Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial, PMID: 32305093
Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial, PMID: 32888452
Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial, PMID: 30522969
Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial, PMID: 27345636
Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions, PMID: 24401022
Obinutuzumab, PMID: 29999701
Immunochemotherapy With Obinutuzumab or Rituximab for Previously Untreated Follicular Lymphoma in the GALLIUM Study: Influence of Chemotherapy on Efficacy and Safety, PMID: 29856692
Comparison of acalabrutinib plus obinutuzumab, ibrutinib plus obinutuzumab and venetoclax plus obinutuzumab for untreated CLL: a network meta-analysis, PMID: 32862749
The role of obinutuzumab in the management of follicular lymphoma, PMID: 31538821
Venetoclax-obinutuzumab: harnessing complexity, PMID: 32163557
Acalabrutinib plus Obinutuzumab in Treatment-Naïve and Relapsed/Refractory Chronic Lymphocytic Leukemia, PMID: 31915195
Obinutuzumab or Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Previously Untreated Diffuse Large B-Cell Lymphoma, PMID: 28796588
Obinutuzumab combined with lenalidomide for relapsed or refractory follicular B-cell lymphoma (GALEN): a multicentre, single-arm, phase 2 study, PMID: 31296423
Obinutuzumab vs rituximab for advanced DLBCL: a PET-guided and randomized phase 3 study by LYSA, PMID: 33211799
Overall Survival Benefit in Patients With Rituximab-Refractory Indolent Non-Hodgkin Lymphoma Who Received Obinutuzumab Plus Bendamustine Induction and Obinutuzumab Maintenance in the GADOLIN Study, PMID: 29584548
Prognostic and predictive impact of genetic markers in patients with CLL treated with obinutuzumab and venetoclax, PMID: 32206772
Obinutuzumab plus Lenalidomide (GALEN) for the treatment of relapse/refractory aggressive lymphoma: a phase II LYSA study, PMID: 30291335
Obinutuzumab in follicular lymphoma, PMID: 28276536
Phase 1b study of venetoclax-obinutuzumab in previously untreated and relapsed/refractory chronic lymphocytic leukemia, PMID: 30862645
CD20-TCB with Obinutuzumab Pretreatment as Next-Generation Treatment of Hematologic Malignancies, PMID: 29716920
Zanubrutinib (BGB-3111) plus obinutuzumab in patients with chronic lymphocytic leukemia and follicular lymphoma, PMID: 33022066
Obinutuzumab for the treatment of indolent lymphoma, PMID: 27117452
Ibrutinib, obinutuzumab, and venetoclax in relapsed and untreated patients with mantle cell lymphoma: a phase 1/2 trial, PMID: 33181832
Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: updated results of the CLL11 study, PMID: 25634683
Clinical application of obinutuzumab for treating chronic lymphocytic leukemia, PMID: 31692500
Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma, PMID: 31693429
Obinutuzumab for B-cell malignancies, PMID: 24856933
Obinutuzumab in Combination with Chemotherapy for the First-Line Treatment of Patients with Advanced Follicular Lymphoma : An Evidence Review Group Evaluation of the NICE Single Technology Appraisal, PMID: 30547368
Randomized Phase II Trial Comparing Obinutuzumab (GA101) With Rituximab in Patients With Relapsed CD20+ Indolent B-Cell Non-Hodgkin Lymphoma: Final Analysis of the GAUSS Study, PMID: 26282650
Risk of HBV reactivation in patients with B-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy, PMID: 30341058
MRD response in relapsed/refractory FL after obinutuzumab plus bendamustine or bendamustine alone in the GADOLIN trial, PMID: 31462735
Obinutuzumab: what is there to learn from clinical trials?, PMID: 28584136
Obinutuzumab: A Review in Rituximab-Refractory or -Relapsed Follicular Lymphoma, PMID: 28324270
Phase II Study of Combination Obinutuzumab, Ibrutinib, and Venetoclax in Treatment-Naïve and Relapsed or Refractory Chronic Lymphocytic Leukemia, PMID: 32795224
A randomized, open-label, Phase III study of obinutuzumab or rituximab plus CHOP in patients with previously untreated diffuse large B-Cell lymphoma: final analysis of GOYA, PMID: 32505213
Obinutuzumab in hematologic malignancies: lessons learned to date, PMID: 26190254
Improving CD20 antibody therapy: obinutuzumab in lymphoproliferative disorders, PMID: 30668192
Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples, PMID: 28407142
Impact of obinutuzumab alone and in combination for follicular lymphoma, PMID: 31360086
Obinutuzumab for the treatment of chronic lymphocytic leukemia, PMID: 24983589
Successful Treatment of Patients With Refractory PLA 2 R-Associated Membranous Nephropathy With Obinutuzumab: A Report of 3 Cases, PMID: 32311405
Obinutuzumab-related adverse events: A systematic review and meta-analysis, PMID: 33252145
Obinutuzumab is Effective for the Treatment of Refractory Membranous Nephropathy, PMID: 32954076
Obinutuzumab: a new class of anti-CD20 monoclonal antibody, PMID: 25014645
Role of obinutuzumab in the treatment of chronic lymphocytic leukemia, PMID: 25987688
Obinutuzumab for the treatment of lymphoproliferative disorders, PMID: 22283718
The fading star of obinutuzumab-chlorambucil regimen in patients with comorbidities with chronic lymphocytic leukemia - are we ready for chemo-free immunotherapy approach?, PMID: 32579408
Combinability of epcoritamab CD20-targeting T-cell engager and CD20 antibody-targeted therapies in B-cell non-Hodgkin lymphoma., PMID:40528731
The anti-CD47 antibody magrolimab with obinutuzumab and venetoclax in relapsed or refractory indolent B-cell lymphomas., PMID:40524014
[Obinutuzumab in lupus nephritis: success with effective B cell depletion]., PMID:40522494
A Phase 2 Study of Obinutuzumab Combined with Lenalidomide in Previously Untreated High Tumor Burden Follicular Lymphoma., PMID:40517417
Beyond BCL2 (B cell lymphoma) and BTK (Bruton tyrosine kinase) inhibitors: novel agents and resistance mechanisms for chronic lymphocytic leukemia., PMID:40515863
Chemotherapy-free combination of ibrutinib and obinutuzumab for untreated advanced follicular lymphoma: results of a phase II study from the German Lymphoma Alliance., PMID:40501414
Advances and challenges in leukemia treatment: A focus on monoclonal antibodies and emerging therapies., PMID:40486885
In active lupus nephritis, adding obinutuzumab to standard therapy increased complete renal response rates at 76 wk., PMID:40456159
Short-duration infusion obinutuzumab with venetoclax in chronic lymphocytic leukemia: a prospective observational study., PMID:40453137
Single-Agent and Associated Therapies with Monoclonal Antibodies: What About Follicular Lymphoma?, PMID:40427101
Obinutuzumab in Patients With Active Lupus Nephritis: A Meta-Analysis., PMID:40424616
Obinutuzumab-Induced Inflammatory Bowel Disease-Like Colitis., PMID:40421460
Cardiac Events in Three Phase 3 Randomized Trials Including Acalabrutinib in Chronic Lymphocytic Leukemia., PMID:40413154
Successful treatment of pemphigus vulgaris with obinutuzumab., PMID:40406957
Additive Obinutuzumab Achieves High Remission Rates in Rituximab-Refractory Membranous Nephropathy., PMID:40388905
Early caplacizumab and obinutuzumab enable successful treatment of relapsing thrombotic thrombocytopenic purpura without therapeutic plasma exchange: a case report., PMID:40356910
Phase II study of venetoclax added to bendamustine and obinutuzumab in patients with high-risk follicular lymphoma as front-line therapy: PrE0403., PMID:40355425
Clinical experience and safety of venetoclax in the treatment of patients with chronic lymphocytic leukemia - real-world data from a hemato-oncology center., PMID:40353625
Obinutuzumab as a Promising Treatment for Membranous Nephropathy., PMID:40352879
CRISPRi screening identifies PIKfyve as a co-therapeutic target for obinutuzumab., PMID:40342289
Obinutuzumab is effective for the treatment of rituximab-refractory PLA2R-associated membranous nephropathy., PMID:40336511
[Seronegativity and anti-CD20: When a treatment compromises the diagnosis]., PMID:40312237
Obinutuzumab for treatment of membranous nephropathy in patients positive and negative for the phospholipase A2 receptor antibody: case reports., PMID:40303416
Obinutuzumab in Rituximab-Intolerant Antineutrophil Cytoplasmic Antibody-Associated Vasculitis Patients., PMID:40303206
Immunotherapy for autoimmune encephalitis., PMID:40301313
Atezolizumab, obinutuzumab and venetoclax for the treatment of patients with relapsed/refractory B non-Hodgkin lymphoma: Final analysis of a phase II trial from the LYSA group., PMID:40285420
Osteonecrosis of the Jaw Associated with Obinutuzumab in a Patient with Preceding Follicular Non-Hodgkin's Lymphoma., PMID:40278317
How we manage immune-mediated thrombotic thrombocytopenic purpura after rituximab failure or intolerance., PMID:40270461
Successful Treatment of Ocular Post-Transplant Lymphoproliferative Disorder with Obinutuzumab in an 8-Year-Old Boy Following Kidney Transplant: A Case Report., PMID:40255019
An autopsy for persistent coronavirus disease 2019 pneumonia during follicular lymphoma treatment: A case report., PMID:40254437
Clinical characteristics, treatment patterns and outcomes of patients with follicular lymphoma in Colombia: a real-world evidence cohort study., PMID:40227475
Obinutuzumab and Ofatumumab are More Effective Than Rituximab in the Treatment of Membranous Nephropathy Patients With Anti-Rituximab Antibodies., PMID:40225374
Obinutuzumab-induced Acute Thrombocytopenia Mimicking Immune Thrombocytopenia in a Patient with Follicular Lymphoma., PMID:40222942
Obinutuzumab in focal segmental glomerulonephritis resistant to treatment., PMID:40221341
AMPLIFY: A second-generation BTK inhibitor for fixed-duration therapy in chronic lymphocytic leukemia., PMID:40220751
B cell depletion in lupus nephritis: From disappointments to hopes, despite some concerns., PMID:40220749
A New Pulmonary Nodule in a Patient With a History of Lymphoma., PMID:40210319
Obinutuzumab: a new frontier in the treatment of refractory idiopathic membranous nephropathy., PMID:40205126
Acalabrutinib-Obinutuzumab Improves Survival vs Chemoimmunotherapy in treatment-naive CLL in the 6-year Follow-up of ELEVATE-TN., PMID:40198878
Obinutuzumab in patients with repeated lupus nephritis flares: A case series., PMID:40186329
Glofitamab in refractory or relapsed diffuse large B cell lymphoma after failing CAR-T cell therapy: a phase 2 LYSA study., PMID:40181090
A pilot study to determine the feasibility and safety of pharmacist and nurse driven management of venetoclax ramp-up in patients with chronic lymphocytic leukemia., PMID:40170466
Treatment of nephrotic syndrome with anti-CD20 therapies in pregnancy: a case series and review of the literature., PMID:40148078
Preclinical B cell depletion and safety profile of a brain-shuttled crystallizable fragment-silenced CD20 antibody., PMID:40118783
Zanubrutinib, venetoclax, and obinutuzumab in R/R CLL., PMID:40111334
The role of obinutuzumab in rituximab-refractory membranous nephropathy and minimal change disease., PMID:40104551
Long-term outcomes of chemoimmunotherapy with obinutuzumab/chlorambucil in chronic lymphocytic leukemia., PMID:40101233
Erratum: Phase II Study of Acalabrutinib, Venetoclax, and Obinutuzumab in a Treatment-Naïve Chronic Lymphocytic Leukemia Population Enriched for High-Risk Disease., PMID:40101168
Upfront fixed-duration treatment strategies for chronic lymphocytic leukemia in Arab populations: a position statement from the Gulf region., PMID:40078401
Real-World Effectiveness of Frontline Treatments Among Patients with Chronic Lymphocytic Leukemia: Results from ConcertAI., PMID:40075647