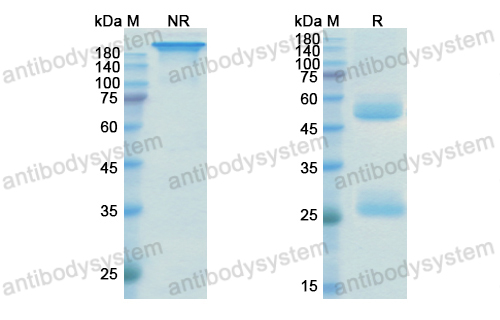
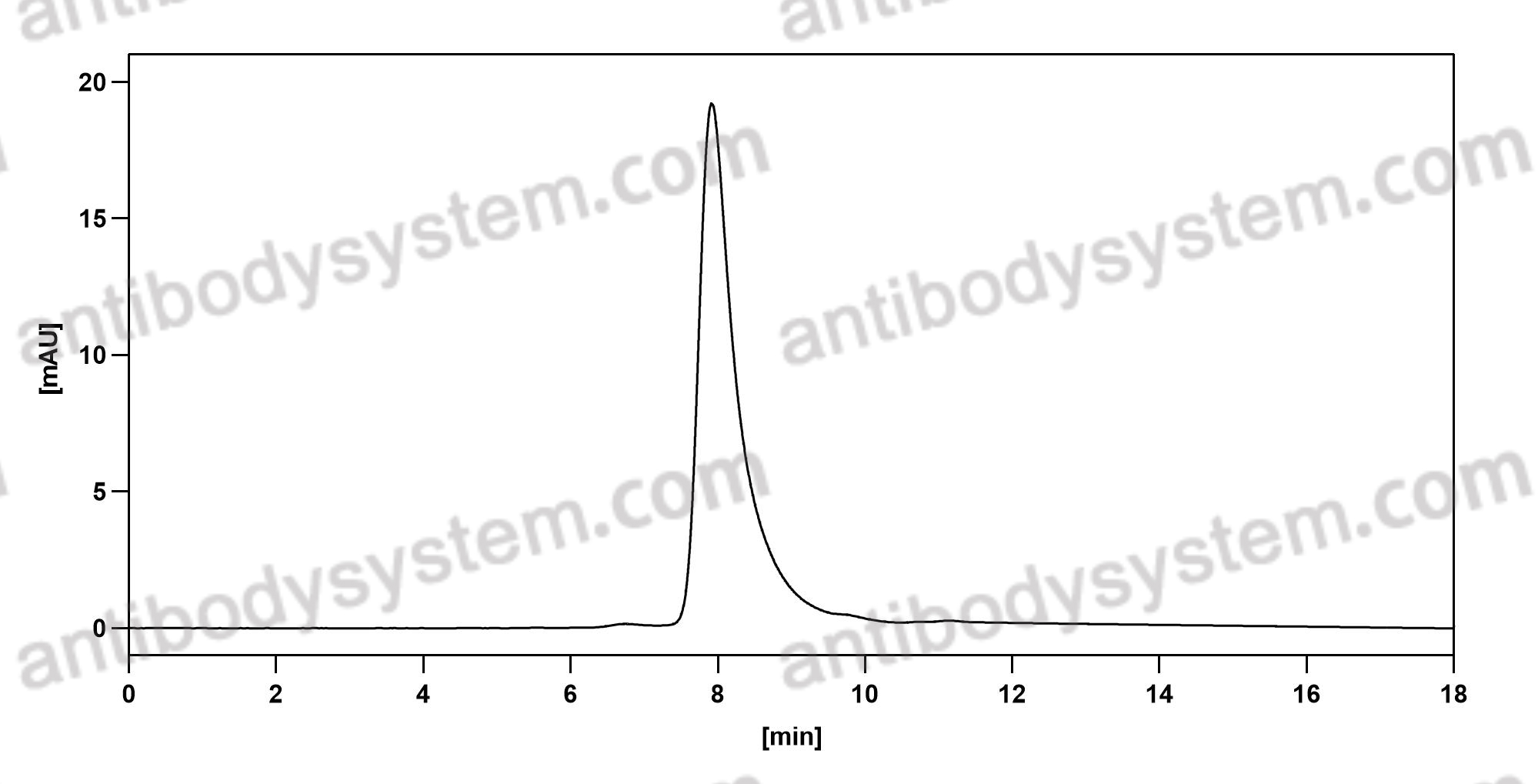
Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis, PMID: 31553833
Ustekinumab as Induction and Maintenance Therapy for Crohn's Disease, PMID: 27959607
Safety of New Biologics (Vedolizumab and Ustekinumab) and Small Molecules (Tofacitinib) During Pregnancy: A Review, PMID: 32562207
Risankizumab versus Ustekinumab for Moderate-to-Severe Plaque Psoriasis, PMID: 28423301
Ustekinumab in the management of Crohn's disease: Expert opinion, PMID: 29610019
IM-UNITI: Three-year Efficacy, Safety, and Immunogenicity of Ustekinumab Treatment of Crohn's Disease, PMID: 31158271
Positioning ustekinumab in moderate-to-severe ulcerative colitis: new kid on the block, PMID: 32027523
Ustekinumab is effective and safe for ulcerative colitis through 2 years of maintenance therapy, PMID: 33086438
Efficacy of Ustekinumab for Inducing Endoscopic Healing in Patients With Crohn's Disease, PMID: 29909019
Ustekinumab is associated with superior effectiveness outcomes compared to vedolizumab in Crohn's disease patients with prior failure to anti-TNF treatment, PMID: 32441396
Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials, PMID: 30097359
Ustekinumab in Pediatric Crohn Disease Patients, PMID: 26854655
[Ustekinumab - Current position], PMID: 30103222
[Ustekinumab], PMID: 31208737
Therapeutic Drug Monitoring With Ustekinumab and Vedolizumab in Inflammatory Bowel Disease, PMID: 29788272
Ustekinumab Safety in Psoriasis, Psoriatic Arthritis, and Crohn's Disease: An Integrated Analysis of Phase II/III Clinical Development Programs, PMID: 30739254
Ustekinumab for Crohn's Disease: Results of the ICC Registry, a Nationwide Prospective Observational Cohort Study, PMID: 31219157
Effects of Ustekinumab on Histologic Disease Activity in Patients With Crohn's Disease, PMID: 31279870
Safety Surveillance for Ustekinumab and Other Psoriasis Treatments From the Psoriasis Longitudinal Assessment and Registry (PSOLAR), PMID: 26151787
Ustekinumab Pharmacokinetics and Exposure Response in a Phase 3 Randomized Trial of Patients With Ulcerative Colitis, PMID: 31816446
Ustekinumab for the treatment of psoriasis: an evidence update, PMID: 30215630
Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial, PMID: 26092291
Effectiveness and safety of ustekinumab induction therapy for 103 patients with ulcerative colitis: a GETAID multicentre real-world cohort study, PMID: 32291786
Real World Experience With Ustekinumab in Children and Young Adults at a Tertiary Care Pediatric Inflammatory Bowel Disease Center, PMID: 31058718
Ustekinumab treatment for hidradenitis suppurativa, PMID: 31638283
Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial, PMID: 28635018
A review of ustekinumab in the treatment of psoriatic arthritis, PMID: 29439608
Long-term efficacy and safety of ustekinumab for Crohn's disease through the second year of therapy, PMID: 29797519
Clinical outcomes with ustekinumab as rescue treatment in therapy-refractory or therapy-intolerant ulcerative colitis, PMID: 32213052
The effectiveness of either ustekinumab or vedolizumab in 239 patients with Crohn's disease refractory to anti-tumour necrosis factor, PMID: 32249966
Association Between Ustekinumab Trough Concentrations and Clinical, Biomarker, and Endoscopic Outcomes in Patients With Crohn's Disease, PMID: 28365485
Efficacy of ustekinumab vs. advanced therapies for the treatment of moderately to severely active ulcerative colitis: a systematic review and network meta-analysis, PMID: 31960724
Safety of ustekinumab or vedolizumab in pregnant inflammatory bowel disease patients: a multicentre cohort study, PMID: 33345331
The safety of ustekinumab for the treatment of psoriatic arthritis, PMID: 28441904
Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study, PMID: 30249507
Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum, PMID: 26641739
Ustekinumab to treat Crohn's disease, PMID: 29042094
Ustekinumab for the treatment of Crohn's disease, PMID: 27450626
Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: Results from the CLEAR study, PMID: 27663079
Efficacy of ustekinumab for active perianal fistulizing Crohn's disease: a systematic review and meta-analysis of the current literature, PMID: 33264569
Effectiveness of ustekinumab dose escalation in Crohn's disease patients with insufficient response to standard-dose subcutaneous maintenance therapy, PMID: 32412134
Secukinumab maintains superiority over ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: 52-week results from a double-blind phase 3b trial (CLARITY), PMID: 32365251
Comorbidity, not patient age, is associated with impaired safety outcomes in vedolizumab- and ustekinumab-treated patients with inflammatory bowel disease-a prospective multicentre cohort study, PMID: 32901983
Maintenance of Efficacy and Safety of Ustekinumab Through One Year in a Phase II Multicenter, Prospective, Randomized, Double-Blind, Placebo-Controlled Crossover Trial of Patients With Active Systemic Lupus Erythematosus, PMID: 31769212
Ustekinumab: A Review in Moderate to Severe Crohn's Disease, PMID: 28528528
Dual biological therapy with anti-TNF, vedolizumab or ustekinumab in inflammatory bowel disease: a systematic review with pool analysis, PMID: 30945576
Ustekinumab for Treating Moderately to Severely Active Crohn's Disease after Prior Therapy: An Evidence Review Group Perspective of a NICE Single Technology Appraisal, PMID: 29192397
Ustekinumab (Stelara) for Crohn's disease, PMID: 28026835
Ixekizumab provides superior efficacy compared with ustekinumab over 52 weeks of treatment: Results from IXORA-S, a phase 3 study, PMID: 29969700
Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis, PMID: 31875504
Influence of Pharmacokinetic-Pharmacodynamic Failure Mechanisms on Outcomes in Biologic Therapy Sequencing in Inflammatory Bowel Disease: A Retrospective Cohort Study., PMID:40531271
Gut Microbial Signatures Associated With Clinical Remission in Inflammatory Bowel Disease Treated With Biologics: A Comprehensive Multi-Cohort Analysis., PMID:40526755
Effectiveness and Safety of Advanced Dual-Targeted Therapy in Refractory Perianal Crohn's Disease., PMID:40504122
Comparative effectiveness of ustekinumab versus infliximab in the management of perianal fistulizing Crohn's disease: a retrospective study in China., PMID:40499579
The Effectiveness of second-and-third line biologics in perianal Crohn's disease - a multicenter propensity score-matched study., PMID:40490896
Wells syndrome: emerging triggers and treatments- an updated systematic review., PMID:40488888
Metabolism and Response to Stress Gene Signatures Reveal Ulcerative Colitis Heterogeneity and Identify Patients With Increased Response to Therapy., PMID:40488582
Impact of Obesity on the Long-Term Outcomes of Advanced Therapies in IBD: A Real-World Study in Taiwan., PMID:40487282
Impact of immediate drug optimization of ustekinumab on medium term targets in Crohn's disease: results from the multicentre retrospective real-life study MUST., PMID:40484747
Safety of guselkumab and ustekinumab treatment in patients with moderate-to-severe plaque psoriasis combined with latent tuberculosis or inactive hepatitis B virus infection: A retrospective multicenter observational study., PMID:40482822
Treatment sequences, outcomes, healthcare utilization, and costs in patients with inflammatory bowel diseases requiring advanced treatment-real world comparative effectiveness from German claims data., PMID:40481399
Interleukin-23 Inhibitors for Inflammatory Bowel Disease: Pivotal Trials and Practical Considerations., PMID:40465057
Guselkumab (Tremfya) - an IL-23 antagonist for Crohn's disease., PMID:40459404
Cancer Incidence in Patients with Ulcerative Colitis Naïve to or Treated with Thiopurine and Targeted Therapies- a cohort study 2007 to 2022 with comparison to the general population., PMID:40455688
Ustekinumab Dose Optimization in Ulcerative Colitis: Is More Always Better?, PMID:40447982
Cross-phenotype genome-wide association study supports shared genetic etiology between skin and gastrointestinal tract diseases., PMID:40441863
Systematic review of comparative studies on emerging psoriasis treatments: comparing biologics with biologics, small molecule inhibitors with small molecule inhibitors, and biologics with small molecule inhibitors., PMID:40439875
Drug survival of IL-23 and IL-17 inhibitors versus other biologics for psoriasis: A British Association of Dermatologists Biologics and Immunomodulators Register cohort study., PMID:40439435
Comparative Efficacy of Ustekinumab and Guselkumab in Improving Itch in Severe Psoriasis Patients., PMID:40432363
Advanced Therapies for Inflammatory Bowel Disease and Risk of Skin Cancer: What's New?, PMID:40427207
The Effects of the Biological Agents Infliximab, Vedolizumab, and Ustekinumab on Intestinal Anastomosis: An Experimental Study in Rats., PMID:40426907
Efficacy and safety of dual-targeted therapy for refractory inflammatory bowel disease: a retrospective case series from three tertiary general hospitals in China., PMID:40421289
Predictors of drug survival of biologics in hidradenitis suppurativa: A systematic review and meta-analysis., PMID:40419221
Short-Term Effectiveness of Ustekinumab in Crohn's Disease: Results from a Real-World Retrospective Multicenter Study in China., PMID:40417421
Use of ustekinumab as the treatment of choice in patient with corticodependent immune-mediated colitis secondary to pembrolizumab., PMID:40409592
Ustekinumab in the Treatment of Crohn's Disease-A Narrative Review on Clinical Efficacy and Safety Profile., PMID:40407511
Evaluating changes in baseline characteristics and drug utilisation pattern in patients with moderate-to-severe psoriasis: findings from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) Cohort., PMID:40402160
Complete Resolution of Pityriasis Rubra Pilaris With Targeted Treatment: A Case Report., PMID:40400847
The Incidence and Management of TNF-α Inhibitor Induced Paradoxical Psoriasis in Children With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis., PMID:40400050
The 'totality of evidence' and 'extrapolation' of SB17, a ustekinumab biosimilar., PMID:40396611
Systematic review and meta-analysis of the efficacy of biologic and targeted synthetic therapies in sarcoidosis., PMID:40393718
Risk of adverse events of psoriasis treatment with biologic agents and new small molecules-BIOBADADERM Registry., PMID:40387427
A systematic review of tumor necrosis factor-α blockers, anti-interleukins, and small molecule inhibitors for dissecting cellulitis of the scalp treatment., PMID:40383754
Induction with upadacitinib in Crohn's disease: real-world experience from an early-access program in Greece., PMID:40371210
Predicting ustekinumab treatment response in Crohn's disease using pre-treatment biopsy images., PMID:40366737
Real-World Insights From Türkiye: Biologic DMARDs Usage in Spondyloarthritis Patients With Chronic Kidney Disease., PMID:40358366
Anti-IL-12/23p40 antibodies for induction of remission in Crohn's disease., PMID:40357993
Cost per remission for mirikizumab versus ustekinumab for moderately to severely active ulcerative colitis treatment from the United States commercial payer perspective., PMID:40351121
Innate and Adaptive Immunity is not Impacted by Inflammatory Bowel Disease Medications in Pregnant Women and Their Offspring., PMID:40349212
Alpha Fail: Ustekinumab to the Rescue After TNFα Failure in Patients with Moderate to Severe Crohn's Disease., PMID:40347351
Second-line strategies after anti-TNF failure in chronically active, moderate-to-severe ulcerative colitis: a retrospective, multicentre cohort study., PMID:40346848
Pyoderma Gangrenosum: A Retrospective Study Comparing TNF-α Inhibitors with Ustekinumab., PMID:40339789
Pharmacokinetic equivalence and comparative safety, tolerability, and immunogenicity of Biocon's ustekinumab (Bmab-1200) with EU-approved and US-licensed reference ustekinumab in healthy subjects: results from the Study to Test pharmacokinetic BioEquivalence of BiosimiLar ustekinumab to SteLARa (STELLAR-1)., PMID:40331766
Ustekinumab is effective in the treatment of linear psoriasis: a case report and literature review., PMID:40330485
The Effectiveness of Medical Therapies for Joint, Skin and Eye Extraintestinal Manifestations in IBD-An Umbrella Review., PMID:40329548
The real-world effectiveness of ustekinumab in patients with ulcerative colitis in the United States., PMID:40327500
One-Year Efficacy of Guselkumab Versus Advanced Therapies for the Treatment of Moderately to Severely Active Crohn's Disease: A Network Meta-Analysis., PMID:40327280
Mirikizumab (Omvoh) - an IL-23 antagonist for Crohn's disease., PMID:40324965
Prevalence of opportunistic infections in Syrian inflammatory bowel disease patients on biologic therapy: a multi-center retrospective cross-sectional study., PMID:40320559