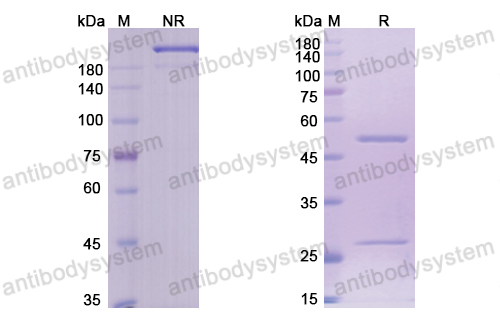
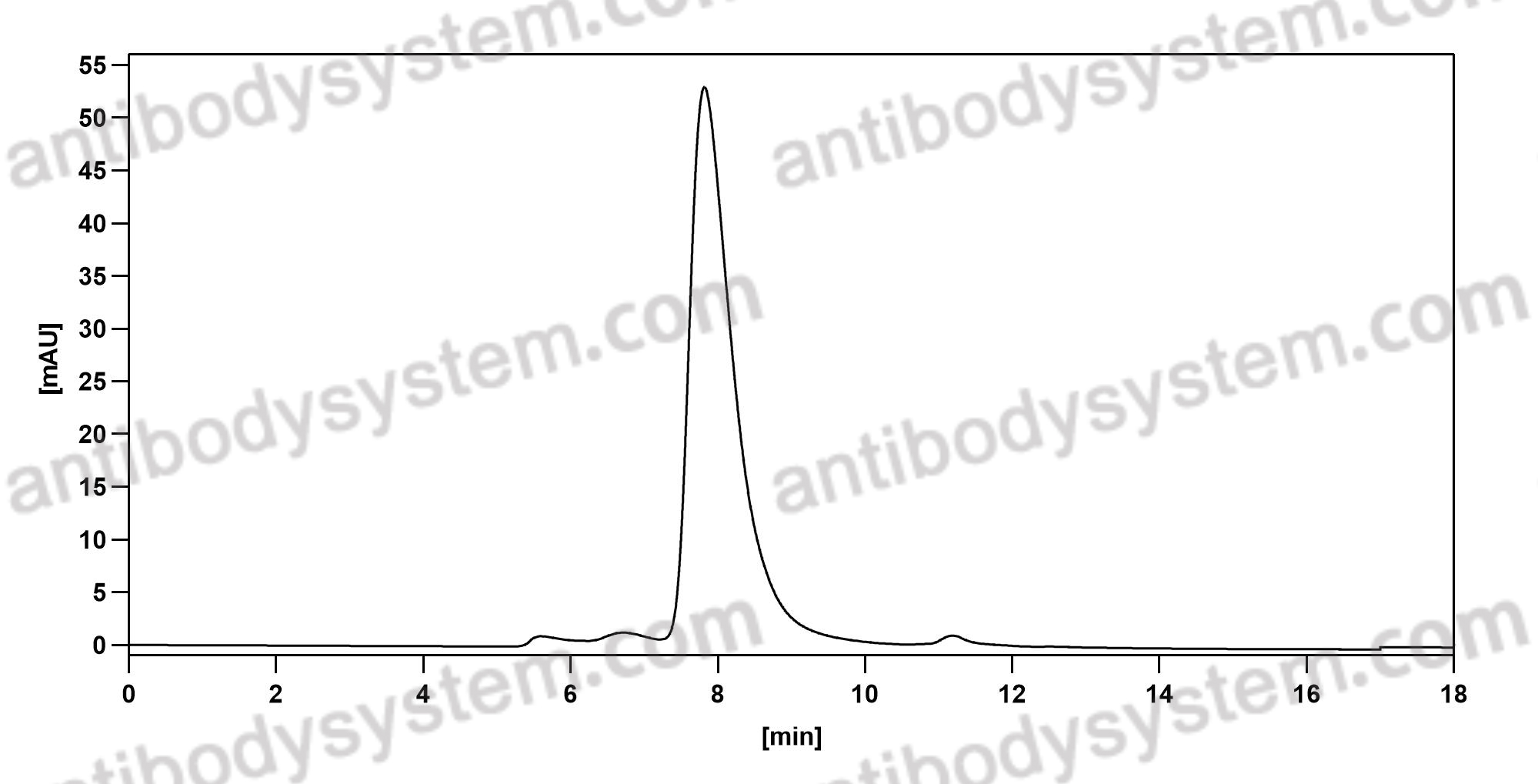
Brentuximab vedotin in T-cell lymphoma, PMID: 30526166
Brentuximab Vedotin: A Review in CD30-Positive Hodgkin Lymphoma, PMID: 28190142
Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial, PMID: 25796459
Brentuximab vedotin with chemotherapy for stage III/IV classical Hodgkin lymphoma: 3-year update of the ECHELON-1 study, PMID: 31945149
Brentuximab vedotin in the treatment of CD30+ PTCL, PMID: 31697814
Brentuximab vedotin or physician's choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial, PMID: 28600132
Brentuximab vedotin for treatment of non-Hodgkin lymphomas: A systematic review, PMID: 28010897
Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma, PMID: 22454421
Brentuximab vedotin, PMID: 22684302
Brentuximab Vedotin in the Treatment of Peripheral T Cell Lymphoma and Cutaneous T Cell Lymphoma, PMID: 32016790
Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin, PMID: 24652992
Brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine for nonbulky limited-stage classical Hodgkin lymphoma, PMID: 31186274
Nivolumab Combined With Brentuximab Vedotin for Relapsed/Refractory Primary Mediastinal Large B-Cell Lymphoma: Efficacy and Safety From the Phase II CheckMate 436 Study, PMID: 31398081
Brentuximab vedotin plus nivolumab as first-line therapy in older or chemotherapy-ineligible patients with Hodgkin lymphoma (ACCRU): a multicentre, single-arm, phase 2 trial, PMID: 33010817
Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma, PMID: 28974506
Brentuximab vedotin and its use in the treatment of advanced Hodgkin's lymphoma, PMID: 32677451
Brentuximab vedotin, PMID: 21410298
Brentuximab vedotin plus bendamustine in relapsed or refractory Hodgkin's lymphoma: an international, multicentre, single-arm, phase 1-2 trial, PMID: 29276022
FDA Approval Summary: Brentuximab Vedotin in First-Line Treatment of Peripheral T-Cell Lymphoma, PMID: 30914464
Brentuximab vedotin for paediatric relapsed or refractory Hodgkin's lymphoma and anaplastic large-cell lymphoma: a multicentre, open-label, phase 1/2 study, PMID: 30290902
Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study, PMID: 22614995
Ipilimumab, nivolumab, and brentuximab vedotin combination therapies in patients with relapsed or refractory Hodgkin lymphoma: phase 1 results of an open-label, multicentre, phase 1/2 trial, PMID: 32853585
Brentuximab vedotin and ESHAP is highly effective as second-line therapy for Hodgkin lymphoma patients (long-term results of a trial by the Spanish GELTAMO Group), PMID: 30657848
Brentuximab vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone as frontline treatment for patients with CD30-positive B-cell lymphomas, PMID: 32414850
Brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine in patients with advanced-stage, classical Hodgkin lymphoma: A prespecified subgroup analysis of high-risk patients from the ECHELON-1 study, PMID: 33462822
Emerging Therapies in Relapsed and Refractory Hodgkin Lymphoma: What Comes Next After Brentuximab Vedotin and PD-1 Inhibition?, PMID: 33409966
Brentuximab vedotin, PMID: 25293772
Frontline brentuximab vedotin in combination with dacarbazine or bendamustine in patients aged ≥60 years with HL, PMID: 29038340
PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin's lymphoma: a non-randomised, open-label, single-centre, phase 2 study, PMID: 25683846
Combining brentuximab vedotin with dexamethasone, high-dose cytarabine and cisplatin as salvage treatment in relapsed or refractory Hodgkin lymphoma: the phase II HOVON/LLPC Transplant BRaVE study, PMID: 32273476
Brentuximab vedotin for relapsed or refractory Hodgkin's lymphoma, PMID: 25848209
Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma, PMID: 27432875
Brentuximab vedotin in Hodgkin's lymphoma, PMID: 22937794
A safety evaluation of brentuximab vedotin for the treatment of Hodgkin lymphoma, PMID: 27139729
Brentuximab vedotin: an anti-CD30 antibody-drug conjugate, PMID: 23515511
An evaluation of brentuximab vedotin as a treatment option for stage III/IV Hodgkin lymphoma, PMID: 31432732
Brentuximab vedotin, PMID: 22212672
Primary prophylaxis with G-CSF may improve outcomes in patients with newly diagnosed stage III/IV Hodgkin lymphoma treated with brentuximab vedotin plus chemotherapy, PMID: 32842815
Brentuximab vedotin in relapsed/refractory Hodgkin lymphoma post-autologous transplant: meta-analysis versus historical data, PMID: 25772232
[Brentuximab vedotin for previously untreated systemic anaplastic large cell lymphoma], PMID: 33308986
Brentuximab vedotin for the treatment of Hodgkin's lymphoma, PMID: 25967932
[Brentuximab vedotin: new treatment for CD30+ lymphomas], PMID: 23831822
Brentuximab vedotin treatment associated with acute and chronic inflammatory demyelinating polyradiculoneuropathies, PMID: 32327451
[Brentuximab vedotin and immune checkpoint inhibitors for the treatment of Hodgkin lymphoma], PMID: 32908053
Brentuximab Vedotin for Relapsed or Refractory Sézary Syndrome, PMID: 33377934
Brentuximab vedotin for lymphoma in paediatric patients, PMID: 30290898
Optimizing the role of brentuximab vedotin in classical Hodgkin lymphoma therapy, PMID: 30504312
Brentuximab vedotin: unexpectedly good response in CD30 - mycosis fungoides, PMID: 31157447
Brentuximab Vedotin (SGN-35), an antibody-drug conjugate for the treatment of CD30-positive malignancies, PMID: 22127011
Brentuximab vedotin for treatment of relapsed or refractory malignant lymphoma: results of a systematic review and meta-analysis of prospective studies, PMID: 25945039
Nivolumab-AVD Versus Brentuximab Vedotin-AVD in Older Patients With Advanced-Stage Classic Hodgkin Lymphoma Enrolled on S1826., PMID:40523203
Classical and biological treatments in mycosis fungoides/Sézary syndrome. New horizons in oncodermatology., PMID:40521064
Successful Re-administration and Extended Dosing of Brentuximab Vedotin Monotherapy for Relapsed Anaplastic Large Cell Lymphoma in a Vulnerable Patient., PMID:40519392
SOHO State of the Art Updates and Next Questions | Update on Tailoring Therapy in T Cell Lymphomas., PMID:40518373
Newly Diagnosed Classical Hodgkin Lymphoma: Optimizing Outcomes in a New Therapeutic Era., PMID:40517538
Peripheral T-Cell Lymphoma: What's Next?, PMID:40517441
Evaluation of the Childhood Hodgkin International Prognostic Score (CHIPS) in High-Risk Pediatric Hodgkin Lymphoma Patients Treated on Children's Oncology Group AHOD1331., PMID:40515508
Tight competition for the first line treatment of Hodgkin's lymphoma: a comprehensive review of practice-changing developments in recent years., PMID:40506833
Treatment of older patients with Hodgkin lymphoma., PMID:40504313
Case Report: Vanishing bile duct syndrome in Hodgkin's lymphoma: a case highlighting jaundice and lymphadenopathy as early clues., PMID:40469301
Final results of a multicentre pilot study evaluating brentuximab vedotin with cyclophosphamide, doxorubicin, etoposide and prednisone (BV-CHEP) for the treatment of aggressive adult T-cell leukaemia/lymphoma., PMID:40459047
Adverse cardiac events associated with antibody drug conjugates in cancer patients: a retrospective analysis on the FAERS database and randomized controlled trials., PMID:40455883
Primary Cutaneous Gamma-Delta T-Cell Lymphoma Presenting With Hemophagocytic Lymphohistiocytosis in a Young Polynesian Male., PMID:40452922
Postmarketing surveillance of brentuximab vedotin for previously untreated Hodgkin lymphoma in Japanese patients., PMID:40451861
Impact of CD3 expression on outcome in pediatric anaplastic large cell lymphoma., PMID:40444097
Clinical Features and Outcomes of Primary Cutaneous Peripheral T-Cell Lymphoma, Not Otherwise Specified, Treated with CHOP-Based Regimens., PMID:40427170
Concomitant treatment with systemic and intralesional brentuximab for resistant CD30+ mycosis fungoides lesions., PMID:40417897
A platform for SpyCatcher conjugation to native antibodies., PMID:40386161
Signal mining and analysis of adverse events of Brentuximab Vedotin base on FAERS and JADER databases., PMID:40378127
Improved efficacy of concurrent anti-PD1 antibody plus AVD versus ABVD in patients with newly diagnosed early unfavorable and advanced stage classic Hodgkin lymphoma: a retrospective matched cohort study., PMID:40372486
Assessment of the Tolerability and Optimal Dosing of the Combination of Brentuximab Vedotin and Lenalidomide in Patients With Relapsed or Refractory T-cell Lymphoma: Results of a Single-centre Phase 1 Dose-escalation Study., PMID:40364805
Anaplastic large cell lymphoma in children and adolescents., PMID:40351161
[Breast implant-associated anaplastic large cell lymphoma that developed 20 years after breast reconstruction and was successfully treated with chemotherapy]., PMID:40350275
Lee HJ, Ramchandren R, Friedman J, et al. Brentuximab vedotin, nivolumab, doxorubicin, and dacarbazine for advanced-stage classical Hodgkin lymphoma. Blood. 2025;145(3):290-299., PMID:40338570
Selecting appropriate therapy for cutaneous T-cell lymphomas (CTCLs): recent advances and the unmet need., PMID:40336318
[Not Available]., PMID:40330314
Nivolumab plus ifosfamide, carboplatin, and etoposide are a highly effective first salvage regimen in high-risk relapsed/refractory Hodgkin lymphoma., PMID:40313510
Brentuximab Vedotin and Nivolumab for Untreated Patients with Hodgkin Lymphoma: Long-term Results., PMID:40311072
Fatal mycosis fungoides, misdiagnosed as contact dermatitis., PMID:40307495
Versatile intact LC-MS method for evaluating the drug-antibody ratio and drug load distribution of antibody-drug conjugates in human plasma., PMID:40267607
Paraneoplastic anti-SRP antibody positive immune-mediated necrotizing myopathy in a young female associated with lymphoma., PMID:40266663
Peripheral Neuropathy Incidence in Children, and Adolescents and Young Adults With Cancer and Medicaid Insurance in California., PMID:40262078
Outcomes of patients with Hodgkin lymphoma receiving Brentuximab Vedotin (BV) as maintenance therapy after ASCT according to previous exposure to BV. A retrospective analysis of the EBMT Lymphoma Working Party in collaboration with GELTAMO, FIL, LYSA, and Turkish Lymphoma Group., PMID:40234724
Nodal Peripheral T-Cell Lymphoma: Therapeutic Challenges and Future Perspectives., PMID:40227698
Retreatment with Brentuximab Vedotin in Patients with Relapsed/Refractory CD30+ Malignancies: A Retrospective Medical Chart Review Study in Spain-The BELIEVE Study., PMID:40227660
Advancements in targeting CD30 for lymphoma therapy: a historical perspective and future directions., PMID:40227173
Drug-induced Guillain-Barré Syndrome: a disproportionality analysis based on the US FDA adverse event reporting system., PMID:40220275
Molecular heterogeneity of CD30+ peripheral T-cell lymphoma with prognostic significance and therapeutic implications: a retrospective multi-centre study., PMID:40215750
Mycosis Fungoides With Small Bowel Involvement Successfully Treated With Brentuximab Vedotin., PMID:40202238
Real-world outcomes of brentuximab vedotin as consolidation therapy after autologous stem cell transplantation in relapsed/refractory Hodgkin lymphoma: A systematic review and meta-analysis., PMID:40200006
Acute Generalized Exanthematous Pustulosis in a Patient Treated With Bendamustine-Brentuximab Vedotin., PMID:40197101
Allogeneic NK cells with a bispecific innate cell engager in refractory relapsed lymphoma: a phase 1 trial., PMID:40186077
Bendamustine supercharge plus brentuximab vedotin as early salvage therapy following failure to obtain complete metabolic remission after two cycles of adriamycin-bleomycin-vinblastine-dacarbazine for classic Hodgkin lymphoma in patients aged ≤ 60 years: Long-term efficacy results of a retrospective multicentre study., PMID:40176291
Next-generation sequencing guides diagnosis and treatment in a complex presentation of ALK-positive anaplastic large-cell lymphoma: a case report., PMID:40161372
Outcomes in patients with classic Hodgkin lymphoma refractory or intolerant to brentuximab vedotin and anti-PD-1 therapy: a real world analysis from 15 U.S. academic centers., PMID:40140364
Therapeutic advances for Cutaneous T Cell Lymphoma., PMID:40131851
Brentuximab vedotin monotherapy is a feasible and effective treatment for older patients with classical Hodgkin lymphoma unsuitable for curative chemotherapy: Results from the prospective GHSG-NLG phase II BVB trial., PMID:40124718
Hodgkin lymphoma presenting as paraneoplastic cerebellar degeneration: A case report., PMID:40115791
Optimizing Brentuximab Vedotin Dosing in Pediatric Patients with Advanced Hodgkin Lymphoma: A Population Pharmacokinetic and Exposure-Response Analysis., PMID:40095373
Systemic Targeted Therapies in Patients with Relapsed/Refractory Advanced Stage Cutaneous T-cell Lymphoma: A Real-world Single-centre Case Series., PMID:40079767