Catalog No.
DXX00401
Expression system
Mammalian Cells
Species reactivity
Bacillus anthracis
Host species
Chimeric
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
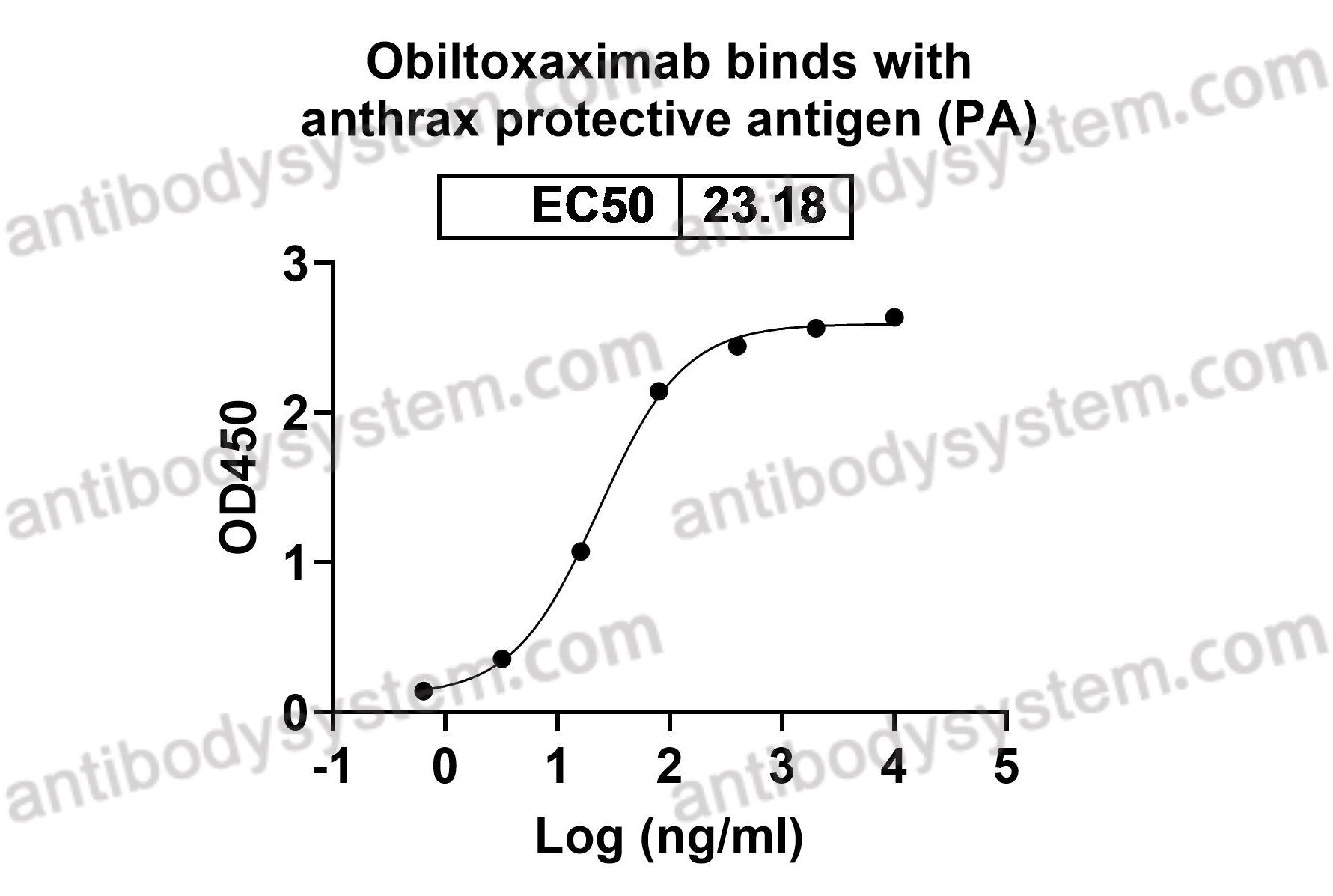
Protective antigen, PA, Anthrax toxins translocating protein, PA-83, PA83, Protective antigen PA-20, PA-20, PA20, Protective antigen PA-63, PA-63, PA63, pagA, pag, pXO1-110, BXA0164, GBAA_pXO1_0164
Concentration
1.5 mg/ml
Endotoxin level
Please contact with the lab for this information.
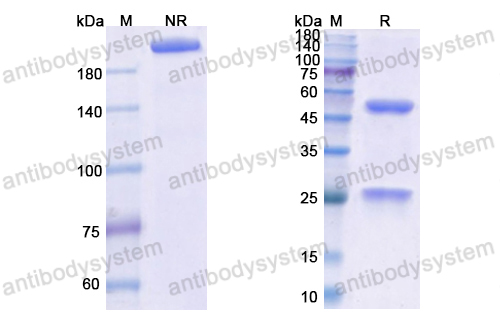
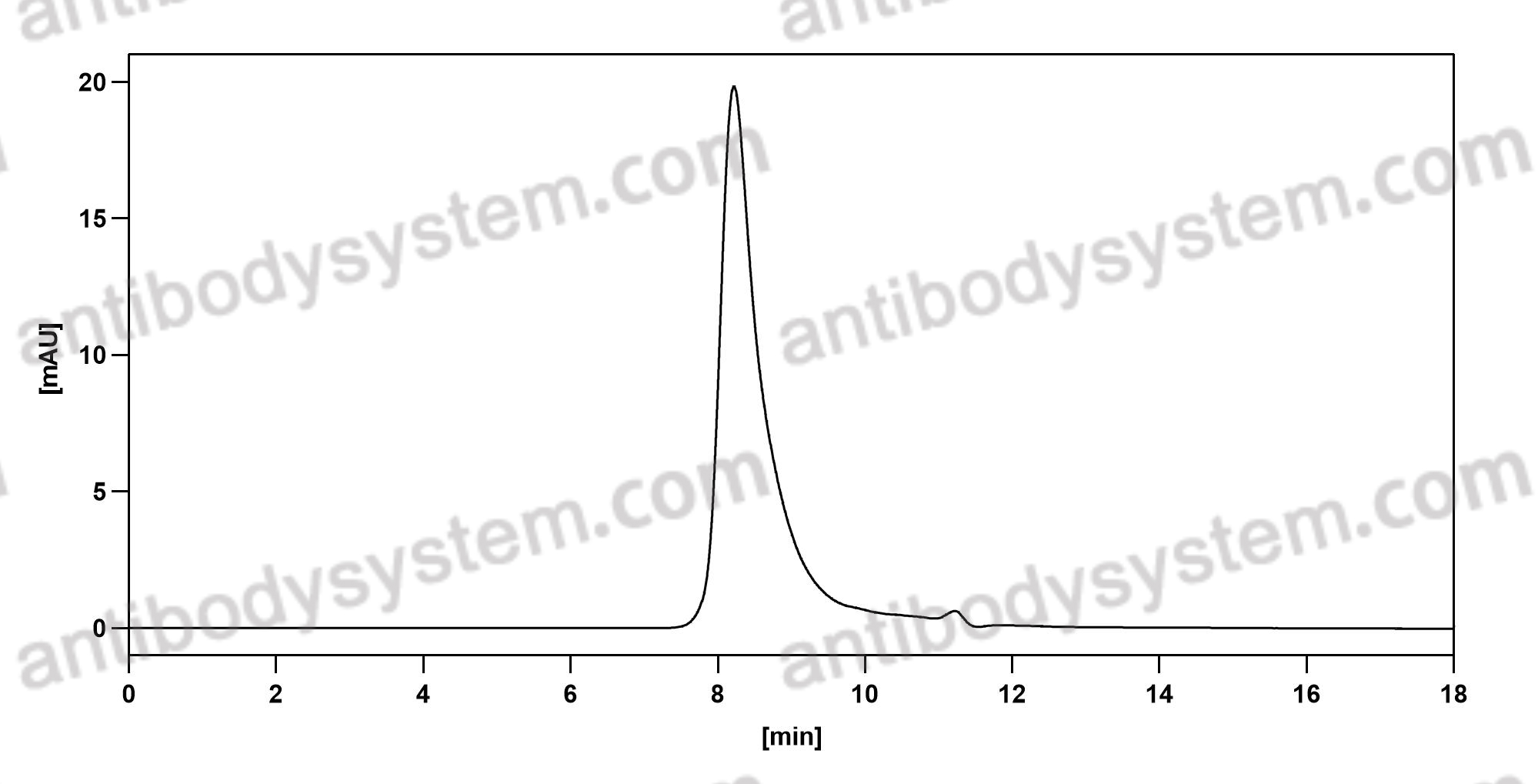
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P13423
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
ETI-204, CAS: 1351337-07-9
Clone ID
Obiltoxaximab
Obiltoxaximab (Anthim) for inhalation anthrax, PMID: 30383733
Obiltoxaximab, PMID: 29999865
Obiltoxaximab: First Global Approval, PMID: 27085536
Obiltoxaximab: Adding to the Treatment Arsenal for Bacillus anthracis Infection, PMID: 28573869
Pharmacokinetics and Tolerability of Obiltoxaximab: A Report of 5 Healthy Volunteer Studies, PMID: 27568215
Obiltoxaximab Prevents Disseminated Bacillus anthracis Infection and Improves Survival during Pre- and Postexposure Prophylaxis in Animal Models of Inhalational Anthrax, PMID: 27431219
Efficacy Projection of Obiltoxaximab for Treatment of Inhalational Anthrax across a Range of Disease Severity, PMID: 27431222
Safety, Pharmacokinetics, and Immunogenicity of Obiltoxaximab After Intramuscular Administration to Healthy Humans, PMID: 29125719
Animal-to-Human Dose Translation of Obiltoxaximab for Treatment of Inhalational Anthrax Under the US FDA Animal Rule, PMID: 27925405
Development of Protective Immunity in New Zealand White Rabbits Challenged with Bacillus anthracis Spores and Treated with Antibiotics and Obiltoxaximab, a Monoclonal Antibody against Protective Antigen, PMID: 29133571
Anthrax Antitoxins, PMID: 31644058
New FDA approved antibacterial drugs: 2015-2017, PMID: 32309599
Antibodies to watch in 2015, PMID: 25484055
Pharmaceutical Approval Update, PMID: 27313431
Antibodies to watch in 2016, PMID: 26651519
Monoclonal Antibodies Against Infectious Microbes: So Long and Too Little!, PMID: 32164518
Alternative pre-approved and novel therapies for the treatment of anthrax, PMID: 27809794
New Drugs 2017, part 3, PMID: 28991071
Efficacy of ETI-204 monoclonal antibody as an adjunct therapy in a New Zealand white rabbit partial survival model for inhalational anthrax, PMID: 25645849
A high-affinity monoclonal antibody to anthrax protective antigen passively protects rabbits before and after aerosolized Bacillus anthracis spore challenge, PMID: 15664918
United States' regulatory approved pharmacotherapies for nuclear reactor explosions and anthrax-associated bioterrorism., PMID:37594915
Pre- and Postlicensure Animal Efficacy Studies Comparing Anthrax Antitoxins., PMID:36251555
A Novel Toll-Like Receptor 2 Agonist Protects Mice in a Prophylactic Treatment Model Against Challenge With Bacillus anthracis., PMID:35369443
Exotoxin-Targeted Drug Modalities as Antibiotic Alternatives., PMID:35099182
Monoclonal Antibodies for Protozoan Infections: A Future Reality or a Utopic Idea?, PMID:34712683
Development of a physiologically-based pharmacokinetic model for ocular disposition of monoclonal antibodies in rabbits., PMID:32876799
Monoclonal Antibodies Against Infectious Microbes: So Long and Too Little!, PMID:32164518
Obiltoxaximab (Anthim) for inhalation anthrax., PMID:30383733
New FDA approved antibacterial drugs: 2015-2017., PMID:32309599
Development of Protective Immunity in New Zealand White Rabbits Challenged with Bacillus anthracis Spores and Treated with Antibiotics and Obiltoxaximab, a Monoclonal Antibody against Protective Antigen., PMID:29133571
Safety, Pharmacokinetics, and Immunogenicity of Obiltoxaximab After Intramuscular Administration to Healthy Humans., PMID:29125719
New Drugs 2017, part 3., PMID:28991071
Obiltoxaximab: Adding to the Treatment Arsenal for Bacillus anthracis Infection., PMID:28573869
Animal-to-Human Dose Translation of Obiltoxaximab for Treatment of Inhalational Anthrax Under the US FDA Animal Rule., PMID:27925405
Alternative pre-approved and novel therapies for the treatment of anthrax., PMID:27809794
Pharmacokinetics and Tolerability of Obiltoxaximab: A Report of 5 Healthy Volunteer Studies., PMID:27568215
Efficacy Projection of Obiltoxaximab for Treatment of Inhalational Anthrax across a Range of Disease Severity., PMID:27431222
Obiltoxaximab Prevents Disseminated Bacillus anthracis Infection and Improves Survival during Pre- and Postexposure Prophylaxis in Animal Models of Inhalational Anthrax., PMID:27431219
Pharmaceutical Approval Update., PMID:27313431
Obiltoxaximab: First Global Approval., PMID:27085536
Antibodies to watch in 2016., PMID:26651519
Efficacy of ETI-204 monoclonal antibody as an adjunct therapy in a New Zealand white rabbit partial survival model for inhalational anthrax., PMID:25645849
Antibodies to watch in 2015., PMID:25484055
A high-affinity monoclonal antibody to anthrax protective antigen passively protects rabbits before and after aerosolized Bacillus anthracis spore challenge., PMID:15664918