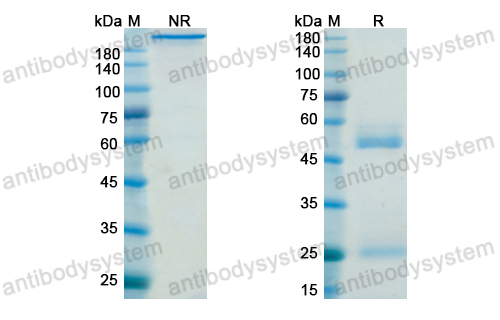
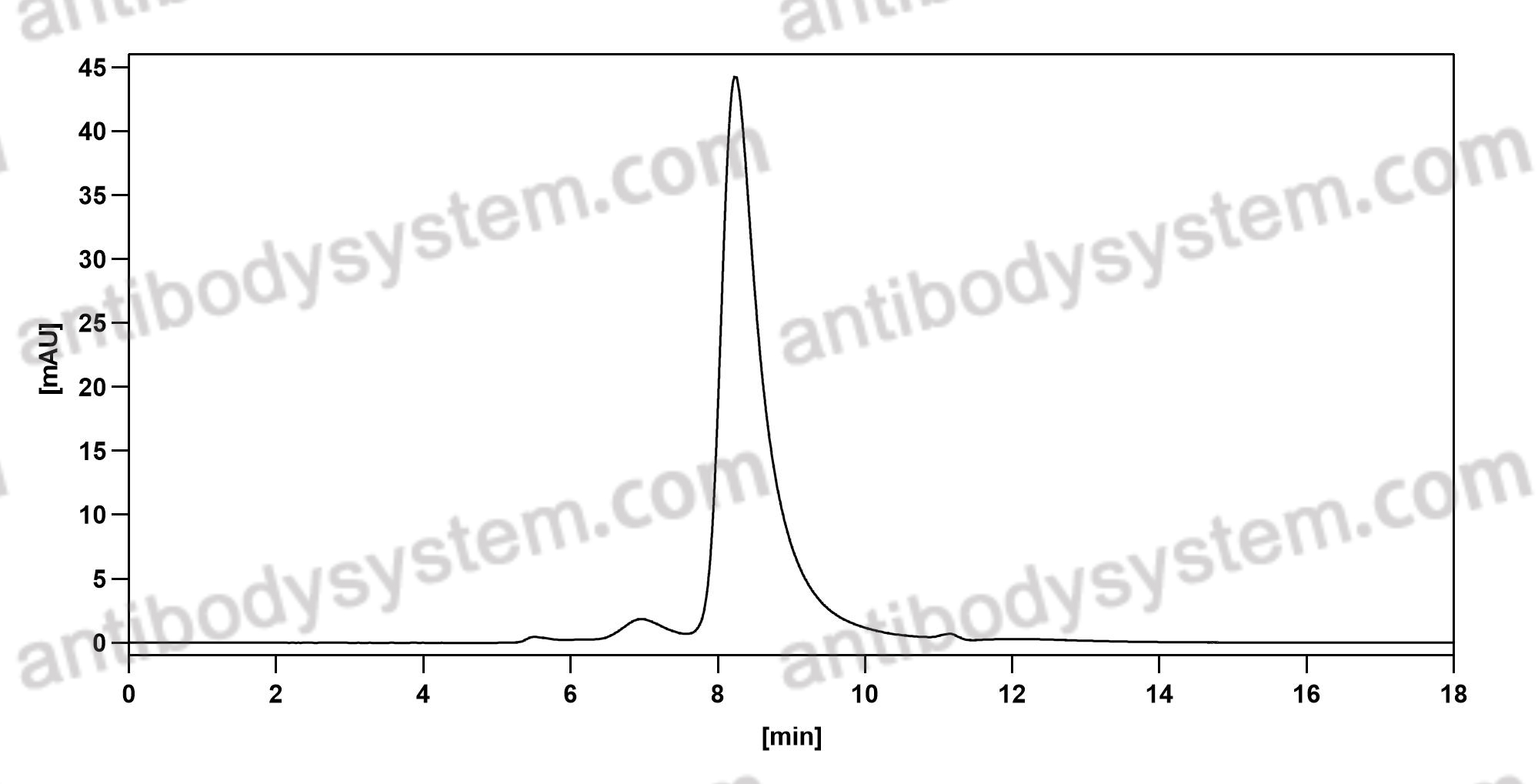
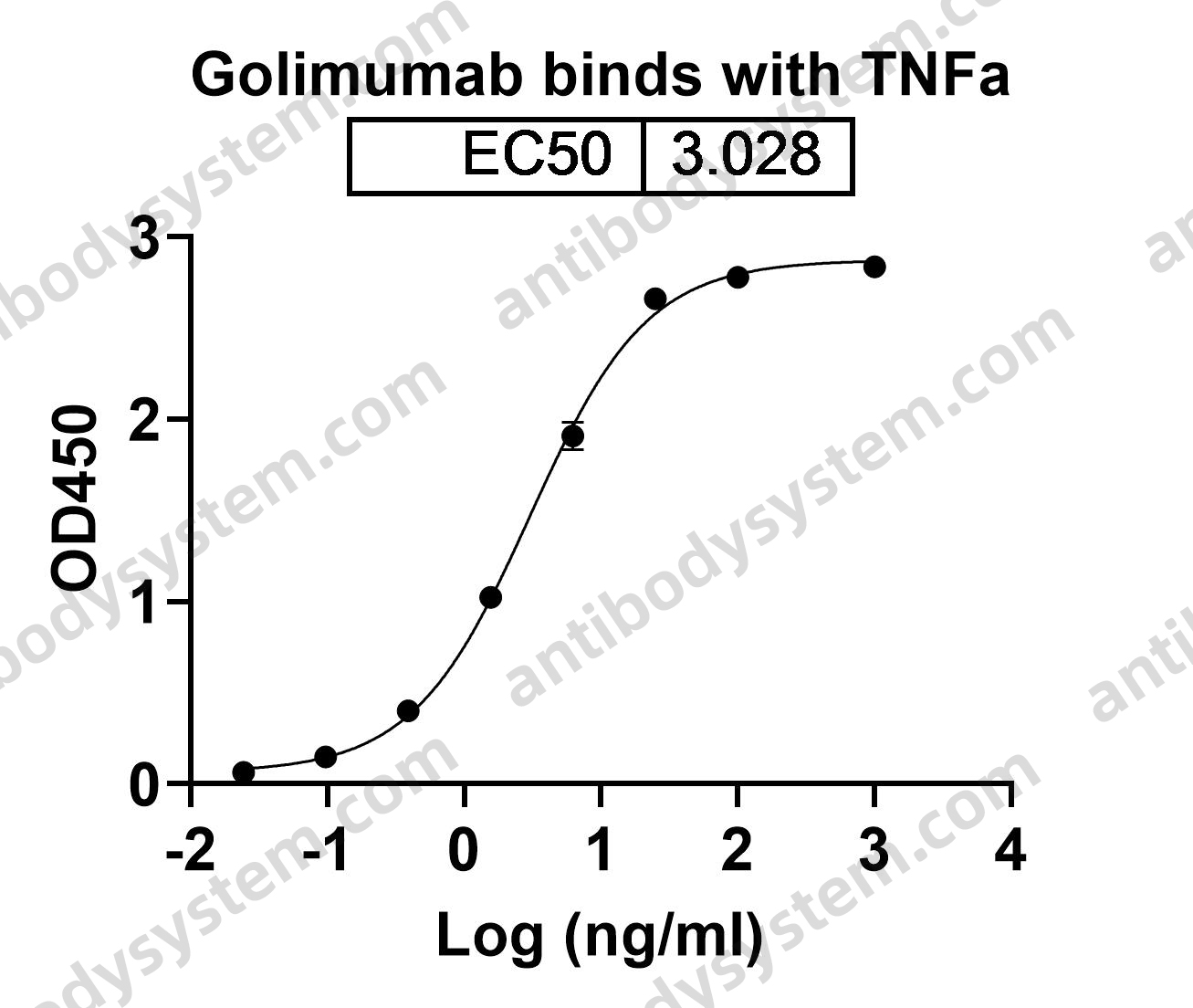
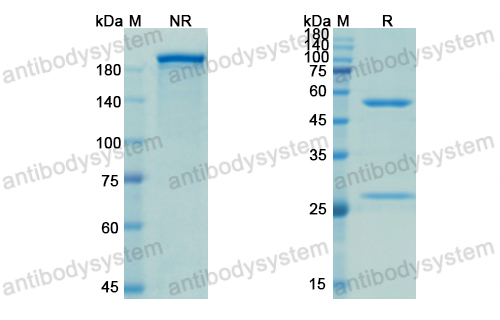
Belimumab: A Review in Systemic Lupus Erythematosus, PMID: 29396833
Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis, PMID: 32937045
Belimumab as Add-on Therapy in Lupus Nephritis, PMID: 32937052
Belimumab and Rituximab in Systemic Lupus Erythematosus: A Tale of Two B Cell-Targeting Agents, PMID: 32695790
Efficacy of belimumab on renal outcomes in patients with systemic lupus erythematosus: A systematic review, PMID: 28147262
A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus, PMID: 22127708
Efficacy of belimumab in Primary Sjögren's syndrome: A systematic review, PMID: 32451263
Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial, PMID: 21296403
Belimumab in lupus nephritis, PMID: 33024302
Safety and efficacy of intravenous belimumab in children with systemic lupus erythematosus: results from a randomised, placebo-controlled trial, PMID: 32699034
Phase II Randomized Trial of Rituximab Plus Cyclophosphamide Followed by Belimumab for the Treatment of Lupus Nephritis, PMID: 32755035
Belimumab, PMID: 31644070
The safety of belimumab for the treatment of systemic lupus erythematosus, PMID: 31657965
Management of Pediatric Systemic Lupus Erythematosus: Focus on Belimumab, PMID: 32612353
Efficacy and Safety of Subcutaneous Belimumab in Systemic Lupus Erythematosus: A Fifty-Two-Week Randomized, Double-Blind, Placebo-Controlled Study, PMID: 28118533
Belimumab, PMID: 29999643
Phase III, multicentre, randomised, double-blind, placebo-controlled, 104-week study of subcutaneous belimumab administered in combination with rituximab in adults with systemic lupus erythematosus (SLE): BLISS-BELIEVE study protocol, PMID: 30898822
Belimumab and low-doses of mycophenolate mofetil as induction therapy of class IV lupus nephritis: case series and literature review, PMID: 29514612
A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea, PMID: 29295825
Belimumab after B cell depletion therapy in patients with systemic lupus erythematosus (BEAT Lupus) protocol: a prospective multicentre, double-blind, randomised, placebo-controlled, 52-week phase II clinical trial, PMID: 31848169
Efficacy and Safety of Belimumab and Azathioprine for Maintenance of Remission in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: A Randomized Controlled Study, PMID: 30666823
Subcutaneous belimumab in the treatment of systemic lupus erythematosus, PMID: 30105936
B cell alterations during BAFF inhibition with belimumab in SLE, PMID: 30593436
Effect of belimumab treatment on renal outcomes: results from the phase 3 belimumab clinical trials in patients with SLE, PMID: 23263865
Safety and Efficacy of Belimumab Plus Standard Therapy for Up to Thirteen Years in Patients With Systemic Lupus Erythematosus, PMID: 30771238
Adding belimumab improves lupus nephritis, PMID: 32994586
The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus, PMID: 29636274
Long-term safety and limited organ damage in patients with systemic lupus erythematosus treated with belimumab: a Phase III study extension, PMID: 31302695
Efficacy of a sequential treatment by belimumab in monogenic systemic lupus erythematosus, PMID: 32910770
Belimumab, PMID: 21532557
Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response, PMID: 22337213
Long-Term Safety and Efficacy of Belimumab in Patients With Systemic Lupus Erythematosus: A Continuation of a Seventy-Six-Week Phase III Parent Study in the United States, PMID: 29409143
Belimumab in kidney transplantation, PMID: 30837144
Belimumab in kidney transplantation, PMID: 30837143
Early Disease and Low Baseline Damage as Predictors of Response to Belimumab in Patients With Systemic Lupus Erythematosus in a Real-Life Setting, PMID: 32275125
Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus, PMID: 22275291
Organ damage in patients treated with belimumab versus standard of care: a propensity score-matched comparative analysis, PMID: 30610066
Systemic lupus erythematosus, PMID: 27306639
Update οn the diagnosis and management of systemic lupus erythematosus, PMID: 33051219
Belimumab: review of use in systemic lupus erythematosus, PMID: 22464040
Subcutaneous formulation of belimumab in treatment of systemic lupus erythematosus: a critical review with focus on safety and satisfaction, PMID: 30538431
Belimumab in the management of systemic lupus erythematosus - an update, PMID: 28460578
Belimumab Treatment for Adults with Systemic Lupus Erythematosus: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines [Internet], PMID: 30379511
Pharmacokinetics of Belimumab in Children With Systemic Lupus Erythematosus, PMID: 33245847
Pharmacoeconomic Review Report: Belimumab (Benlysta): (GlaxoSmithKline Inc.) [Internet], PMID: 33237683
Belimumab: in systemic lupus erythematosus, PMID: 22141386
Impact of belimumab on patient-reported outcomes in systemic lupus erythematosus: review of clinical studies, PMID: 30666173
Systematic review of safety and efficacy of belimumab in treating immune-mediated disorders, PMID: 33368349
Successful treatment of systemic lupus erythematosus pleuropericarditis with belimumab, PMID: 31364982
Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials, PMID: 22550315
Clinical Outcomes of Patients with SLE Treated with Belimumab, Without Versus With Prior Immunosuppressant Use: a US Claims Database Study., PMID:40528076
Belimumab to Aid Pre-Transplant Immunological Risk-Stratification by Uncovering Broader HLA-Specific Memory B-Cell Profiles., PMID:40522199
Efficacy and safety of belimumab in lupus nephritis patients with nephrotic syndrome: a single-centre observational retrospective study., PMID:40463808
Rare case of systemic lupus with manifestation of FS Jaccoud arthropathy: a case report and literature review., PMID:40461984
Alternation of anti-NMDA receptor subunit GluN2 antibody in a patient with SLE who promptly developed anxiety after belimumab., PMID:40455606
Enhancing systemic lupus erythematosus treatment outcomes with an early initiation of belimumab: insights from a multicenter retrospective study within the first five years., PMID:40442811
Management of childhood-onset systemic lupus erythematosus (cSLE) over the last two decades in Spain., PMID:40426215
Efficacy and safety of belimumab in refractory and newly diagnosed active lupus nephritis patients: a real-world observational study., PMID:40416394
Dual B-cell targeting in systemic lupus erythematosus: The role of combined and sequential therapy with rituximab and belimumab., PMID:40414587
Infectious complications of Belimumab with standard care in systemic lupus erythematosus: a systematic review and meta-analysis., PMID:40398101
Chilblains With Tumid Lupus Features in a Patient With Sjögren's Syndrome: A Case Report., PMID:40385803
Case of class II lupus nephritis with nephrotic features in the context of belimumab: Intersecting clinical challenge with academic initiative to emphasize social barriers in care., PMID:40371310
Differences in dynamics of specific anti-nuclear antibodies and their susceptibility to B cell targeting treatment in Systemic Lupus Erythematosus., PMID:40356198
Efficacy and safety of belimumab versus rituximab for refractory immune thrombocytopenia in patients with connective tissue disease., PMID:40355343
Comparative efficacy and acceptability of novel biologics in the treatment of myasthenia gravis: systematic review and network meta-analysis of randomized trials., PMID:40346603
Efficacy and Safety of Biologics and Immunosuppressants in Maintenance Therapy for Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: A Network Meta-Analysis., PMID:40325583
The effect of belimumab on mucocutaneous and vasculitis manifestations in patients with systemic lupus erythematosus: A large pooled post hoc analysis., PMID:40322925
Anifrolumab in childhood-onset systemic lupus erythematosus: a promising option after belimumab failure., PMID:40312207
Role of B-Cell Inhibition in Autoimmune Hepatitis: Lessons Learnt From Systemic Lupus Erythematosus., PMID:40276396
Outcomes of patients with systemic lupus erythematosus treated with belimumab: a post hoc efficacy analysis of five phase III clinical trials by British Isles Lupus Assessment Group-based Combined Lupus Assessment criteria., PMID:40274305
Role of belimumab in recurrent spontaneous abortions amongst patients with lymphocyte dysfunction: a retrospective case-control study., PMID:40259254
Predicting EQ-5D full health state in systemic lupus erythematosus using machine learning algorithms., PMID:40256631
Unsupervised machine learning identifies distinct systemic lupus erythematosus patient endotypes with differential response to belimumab., PMID:40244828
Heterogeneity of peripheral immune cell landscape in systemic lupus erythematosus patients after belimumab treatment., PMID:40242921
Belimumab in Childhood Onset SLE: Update and Evidence., PMID:40195647
Disease-Modifying Therapies in Lupus Nephritis: A Narrative Review Evaluating Currently Used Pharmacologic Agents., PMID:40186747
Evaluation of the efficacy and safety of belimumab and telitacicept in patients with systemic lupus erythematosus: results from a retrospective, observational study., PMID:40172681
[Lupus nephritis - does a lot help a lot?]., PMID:40164100
Integrative miRNA-mRNA profiling uncovers mechanisms of belimumab action in systemic lupus erythematosus., PMID:40160819
Biological therapies in thrombocytopenia and primary antiphospholipid syndrome: where are we now?, PMID:40151064
Evaluation of biological therapies in autoimmune hepatitis: A case-based systematic review., PMID:40123748
Kidney transplantation in Lupus Nephritis: a comprehensive review of challenges and strategies., PMID:40121458
Comparative efficacy and safety of anifrolumab for belimumab-experienced and biologic-naïve patients with systemic lupus erythematosus: Results from the Kyushu Collagen Disease Network for Systemic Lupus Erythematosus (KCDN-SLE) registry., PMID:40090613
Belimumab efficacy in mucocutaneous lupus erythematosus: a large post-hoc analysis from five phase III clinical trials., PMID:40085009
Transcriptome analysis to decipher the molecular underpinnings of response to treatment in systemic lupus erythematosus., PMID:40081913
Belimumab patient profile in Spain: evolution during the last decade and future directions., PMID:40077897
Systemic lupus erythematosus: one year in review 2025., PMID:40072872
Evolution and trajectory of B-cell targeted therapies in rheumatic diseases., PMID:40058377
Disease-Modifying Antirheumatic Drug Therapy of Inflammatory Rheumatic Diseases in End-Stage Kidney Disease., PMID:40049155
A focused report on IFN-1 targeted therapies for lupus erythematosus., PMID:40047795
Belimumab concentrations and immunogenicity in relation to drug effectiveness and safety in SLE within a Swedish real-world setting., PMID:40037576
Real world use of belimumab in patients with lupus nephritis., PMID:40029832
A Review of Therapeutics for the Treatment of Lupus., PMID:40016631
Treatment of thrombotic microangiopathy associated with systemic lupus erythematosus with low-dose rituximab as an induction agent and belimumab as a maintenance agent., PMID:40001033
Renal recovery from membranous lupus nephritis with antineutrophil cytoplasmic antibody-associated glomerulonephritis with belimumab., PMID:39995263
A Case Report of Severe Systemic Lupus Erythematosus with Anti-centromere Antibody., PMID:39993749
Treat-to-target in SLE: is serology important? Results from an integrated analysis of five randomized clinical trials of belimumab., PMID:39985454
Memory B cells and their transcriptomic profiles associated with belimumab resistance in systemic lupus erythematosus in the maintenance phase., PMID:39975549
Real-World Effectiveness of Intravenous Belimumab on Clinical Outcomes in Patients With Systemic Lupus Erythematosus in Saudi Arabia: The OBSErve Observational Study., PMID:39973976