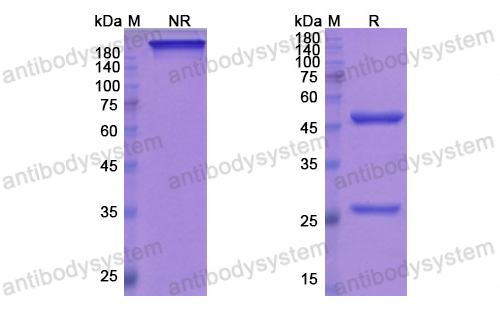
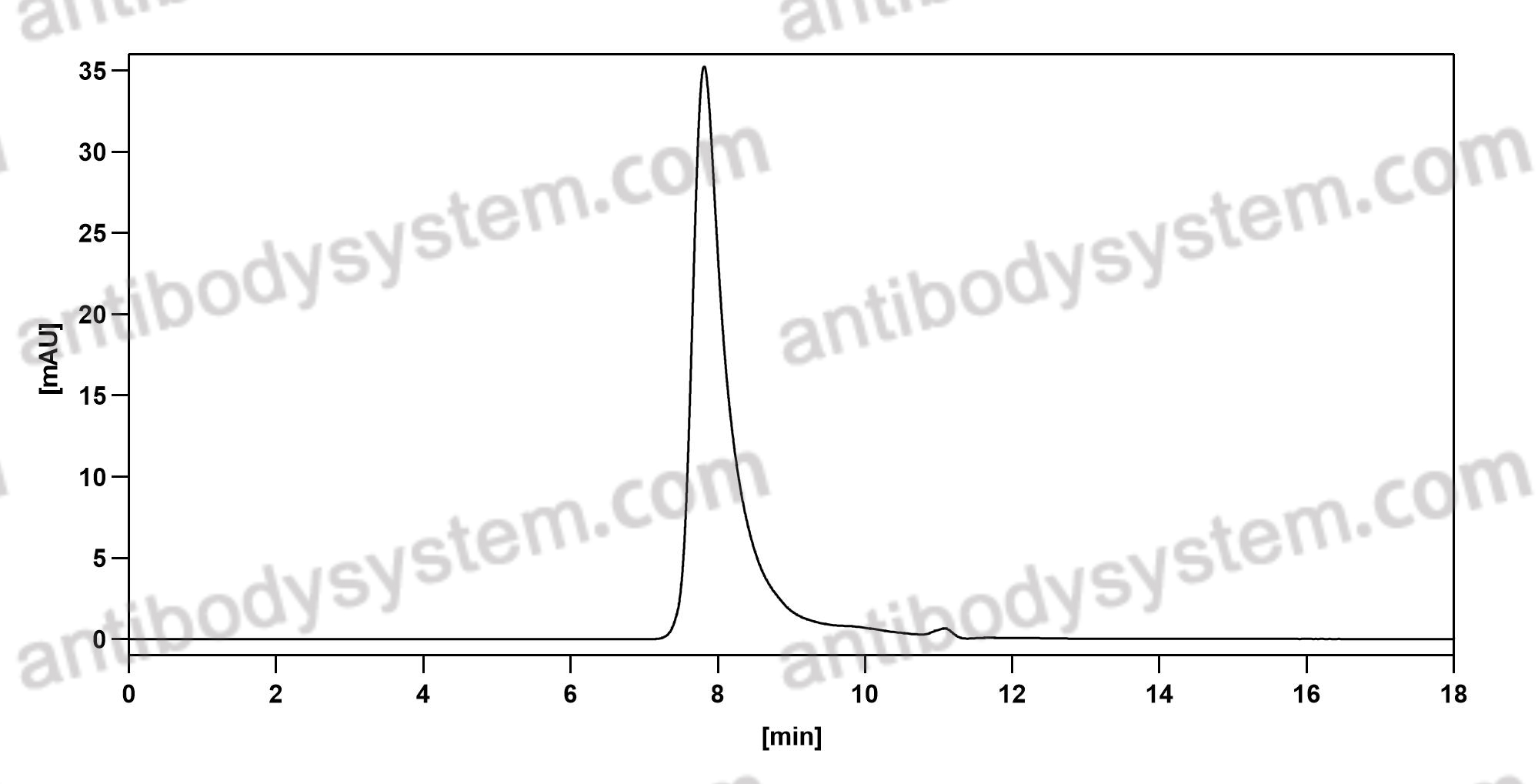
Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis, PMID: 28514601
Mepolizumab treatment in patients with severe eosinophilic asthma, PMID: 25199059
Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma, PMID: 32034960
Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial, PMID: 22901886
Mepolizumab effectiveness and identification of super-responders in severe asthma, PMID: 32139455
Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma, PMID: 25199060
Mepolizumab for Eosinophilic Chronic Obstructive Pulmonary Disease, PMID: 28893134
Efficacy and safety of mepolizumab in hypereosinophilic syndrome: A phase III, randomized, placebo-controlled trial, PMID: 32956756
Real-world mepolizumab in the prospective severe asthma REALITI-A study: initial analysis, PMID: 32817259
Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma, PMID: 30359681
The clinical benefit of mepolizumab replacing omalizumab in uncontrolled severe eosinophilic asthma, PMID: 31049972
Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial, PMID: 28687232
Mepolizumab (Nucala) [Internet], PMID: 28121100
Long-term Safety and Clinical Benefit of Mepolizumab in Patients With the Most Severe Eosinophilic Asthma: The COSMEX Study, PMID: 31447130
Long-Term Clinical Outcomes of High-Dose Mepolizumab Treatment for Hypereosinophilic Syndrome, PMID: 29751154
Subcutaneous mepolizumab in children aged 6 to 11 years with severe eosinophilic asthma, PMID: 31502421
The spectrum of therapeutic activity of mepolizumab, PMID: 31424304
Long-term safety and pharmacodynamics of mepolizumab in children with severe asthma with an eosinophilic phenotype, PMID: 31425781
An evaluation of mepolizumab for the treatment of severe asthma, PMID: 31009582
Mepolizumab for the treatment of eosinophilic granulomatosis with polyangiitis, PMID: 31146595
Evaluation of clinical benefit from treatment with mepolizumab for patients with eosinophilic granulomatosis with polyangiitis, PMID: 30578883
Mepolizumab in a population with severe eosinophilic asthma and corticosteroid dependence: results from a French early access programme, PMID: 32241829
Real-World Effectiveness and the Characteristics of a "Super-Responder" to Mepolizumab in Severe Eosinophilic Asthma, PMID: 32275980
Benralizumab after sub-optimal response to mepolizumab in severe eosinophilic asthma, PMID: 33300186
Mepolizumab for the treatment of eosinophilic granulomatosis with polyangiitis, PMID: 29637936
Mabs for treating asthma: omalizumab, mepolizumab, reslizumab, benralizumab, dupilumab, PMID: 32401603
Mepolizumab in the treatment of eosinophilic chronic obstructive pulmonary disease, PMID: 31496677
Mepolizumab as Possible Treatment for Allergic Bronchopulmonary Aspergillosis: A Review of Eight Cases, PMID: 32923277
Mepolizumab: First Global Approval, PMID: 26603873
[CME: Mepolizumab, an Additional Therapeutic Agent for Severe Asthma], PMID: 30326819
Mepolizumab and exacerbations of refractory eosinophilic asthma, PMID: 19264686
Mepolizumab for the treatment of severe eosinophilic asthma, PMID: 28645995
Mepolizumab: A Review in Eosinophilic Asthma, PMID: 27311938
The safety of mepolizumab for the treatment of asthma, PMID: 28116937
Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis, PMID: 21958585
Mepolizumab for the prevention of chronic obstructive pulmonary disease exacerbations, PMID: 30570418
Role of Biologics in Asthma, PMID: 30525902
Treatment of patients with the hypereosinophilic syndrome with mepolizumab, PMID: 18344568
Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial, PMID: 28395936
The relationship between Feno and effectiveness of mepolizumab and benralizumab in severe eosinophilic asthma, PMID: 33486140
Severe eosinophilic asthma with nasal polyposis: A phenotype for improved sinonasal and asthma outcomes with mepolizumab therapy, PMID: 32084443
Long-term Efficacy and Safety of Mepolizumab in Patients With Severe Eosinophilic Asthma: A Multi-center, Open-label, Phase IIIb Study, PMID: 27553751
Mepolizumab for Treating Severe Eosinophilic Asthma: An Evidence Review Group Perspective of a NICE Single Technology Appraisal, PMID: 28933002
Mepolizumab and eosinophil-mediated disease, PMID: 19929788
Mepolizumab-based therapy in asthma: an update, PMID: 26110690
Mepolizumab versus placebo for asthma, PMID: 26214266
Mepolizumab for allergic bronchopulmonary aspergillosis: Report of 20 cases from the Belgian Severe Asthma Registry and review of the literature, PMID: 32268213
Mepolizumab for Eosinophilic COPD, PMID: 29446292
Mepolizumab for Eosinophilic COPD, PMID: 29446291
Mepolizumab for Eosinophilic COPD, PMID: 29443666
Cost-effectiveness of mepolizumab vs anti-interleukin-5/5r biologic therapies for the treatment of adults with severe asthma with an eosinophilic phenotype: a Chilean healthcare system perspective., PMID:40528625
Th2-High Severe Asthma with Hypereosinophilia in the Spectrum of Type 2 Inflammatory Diseases., PMID:40508151
Induction therapy with mepolizumab for cardiac involvement in eosinophilic granulomatosis with polyangiitis., PMID:40494150
Aspirin-Exacerbated Respiratory Disease in the era of biologics., PMID:40490219
Wells syndrome: emerging triggers and treatments- an updated systematic review., PMID:40488888
Steroid-sparing benefits of biologic use in hypereosinophilic syndrome and substantial disease burden across subtypes., PMID:40486501
Advancements in Biologic Therapies for Pediatric Asthma: Emerging Therapies and Future Directions., PMID:40486460
A rare case of sudden blindness following mepolizumab withdrawal in EGPA., PMID:40485495
A nationwide experience of biological treatments in children with eosinophilic esophagitis., PMID:40483369
A case of hereditary alpha tryptasemia and presumptive eosinophilic granulomatosis with polyangiitis., PMID:40458680
Two unique cases of eosinophilic granulomatosis with polyangiitis in childhood treated with anti-interleukin-5 therapy: infantile-onset and submandibular salivary gland involvement., PMID:40457436
Mepolizumab Prevents COPD Exacerbations in Trial., PMID:40445610
Mepolizumab Improves Histologic Severity as Measured by the EoEHSS in Adolescents and Adults With Eosinophilic Oesophagitis in a Multicenter, Randomised, Double-Blind, Placebo-Controlled Clinical Trial., PMID:40443051
Mepolizumab as an Effective Alternative to Immunosuppressive and Teratogenic Therapies for the Early Treatment of EGPA: A Case Report., PMID:40440108
Eosinophilic Granulomatosis With Polyangiitis Presenting as Bilateral Orbital Inflammation., PMID:40438859
Real-World Effectiveness and Safety of Mepolizumab in Severe Eosinophilic Asthma: Insights From the Korean Severe Asthma Registry (KoSAR)., PMID:40414814
Long-term safety and effectiveness of mepolizumab for patients with bronchial asthma in routine clinical practice in Japan - final report of special drug use investigation., PMID:40398189
Hypereosinophilia: clinical and therapeutic approach in 2025., PMID:40396537
A Rare Case of Autoimmune Pulmonary Alveolar Proteinosis Developing During the Course of Eosinophilic Granulomatosis With Polyangiitis., PMID:40395486
Eosinophilic granulomatosis with polyangiitis: current status and future perspectives., PMID:40383090
Long-Term Longitudinal Analysis of Pulmonary Function Before and After Biological Therapy in Severe Asthma., PMID:40373751
Drug evaluation: mepolizumab in chronic obstructive pulmonary disease., PMID:40366762
Biologic Therapies for Severe Asthma: Current Insights and Future Directions., PMID:40364184
Small Airways Disease as a Novel Target for Mepolizumab in Asthma-The SASAM Prospective Real-Life Study., PMID:40363960
Requalification of patients with severe asthma for biological therapy-Practical 'ReQuaBi' rate decision scheme based on the analytical model., PMID:40348613
Reappraisal of Biologic Efficacy from Phase 3 Trials in Refractory Chronic Rhinosinusitis and Nasal Polyps., PMID:40340024
Relapse of Eosinophilic Granulomatosis With Polyangiitis (EGPA) Despite Maintenance Treatment With Low-Dose Mepolizumab., PMID:40337294
The effect of mepolizumab dosage form on treatment outcomes in severe asthma., PMID:40330778
Switching from multiple-inhaler triple therapy to single, extrafine-inhaler triple therapy in severe refractory asthma with EGPA: beyond control. Case report and review of the literature., PMID:40322221
Mepolizumab in COPD - If at First You Don't Succeed., PMID:40305854
Mepolizumab for COPD with Eosinophilic Phenotype following Hospitalization., PMID:40305842
Mepolizumab in COPD - If at First You Don't Succeed., PMID:40305719
Mepolizumab to Prevent Exacerbations of COPD with an Eosinophilic Phenotype., PMID:40305712
Comparative Insights on IL-5 Targeting with Mepolizumab and Benralizumab: Enhancing EGPA Treatment Strategies., PMID:40305320
Efficacy of tiotropium bromide on spirometric measurements and control of asthma in real life: data from a 1-year clinical follow-up., PMID:40304436
[Multidisciplinary expert consensus on diagnosis and treatment of eosinophilic granulomatosis with polyangiitis (2025 Edition)]., PMID:40300867
Improving patient outcomes: Mepolizumab's impact in IL-5-mediated diseases., PMID:40296395
Mepolizumab in the treatment of eosinophilic cystitis: a pooled case review., PMID:40293614
Long-term efficacy and safety of different biologics in treatment of chronic rhinosinusitis with nasal polyps: A network meta-analysis., PMID:40286594
Impact of mepolizumab on the AAV-PRO questionnaire in eosinophilic granulomatosis with polyangiitis: data from a European multicentre study., PMID:40286311
Biologic Agents in Idiopathic Hypereosinophilic Syndrome., PMID:40283978
New Therapeutic Challenges in Pediatric Gastroenterology: A Narrative Review., PMID:40281872
Sinonasal Intervention Reduces the Need for Pressure Equalization Tube Placement in Atopic Adults., PMID:40265713
Diffusion dimensionality modeling of subcutaneous/intramuscular absorption of antibodies and long-acting injectables., PMID:40263185
Efficacy of Biologics in Reducing Exacerbations Requiring Hospitalization or an Emergency Department Visit in Patients with Moderate or Severe, Uncontrolled Asthma., PMID:40261563
Circulating Mast Cell Progenitors Are Depleted by Benralizumab in Severe Asthma., PMID:40251899
Comparative Effectiveness of Dupilumab Versus Mepolizumab and Benralizumab in Asthma: A Multinational Retrospective Cohort Study., PMID:40250559
Mepolizumab in severe uncontrolled CRSwNP: a real-life multicentre study in Northeast Italy., PMID:40249916
Early Intervention with Mepolizumab, Corticosteroids, and Intravenous Immunoglobulin for Dupilumab-Triggered Eosinophilic Granulomatosis with Polyangiitis: A Case Report., PMID:40248105
Effectiveness of tezepelumab in preventing relapse of eosinophilic granulomatosis with polyangiitis: A case report., PMID:40239417