Catalog No.
EVV04701
Expression system
Mammalian Cells
Species
Varicella-zoster virus (VZV/HHV-3)
Protein length
Met1-Tyr538
Predicted molecular weight
61.64 kDa
Nature
Recombinant
Endotoxin level
Please contact with the lab for this information.
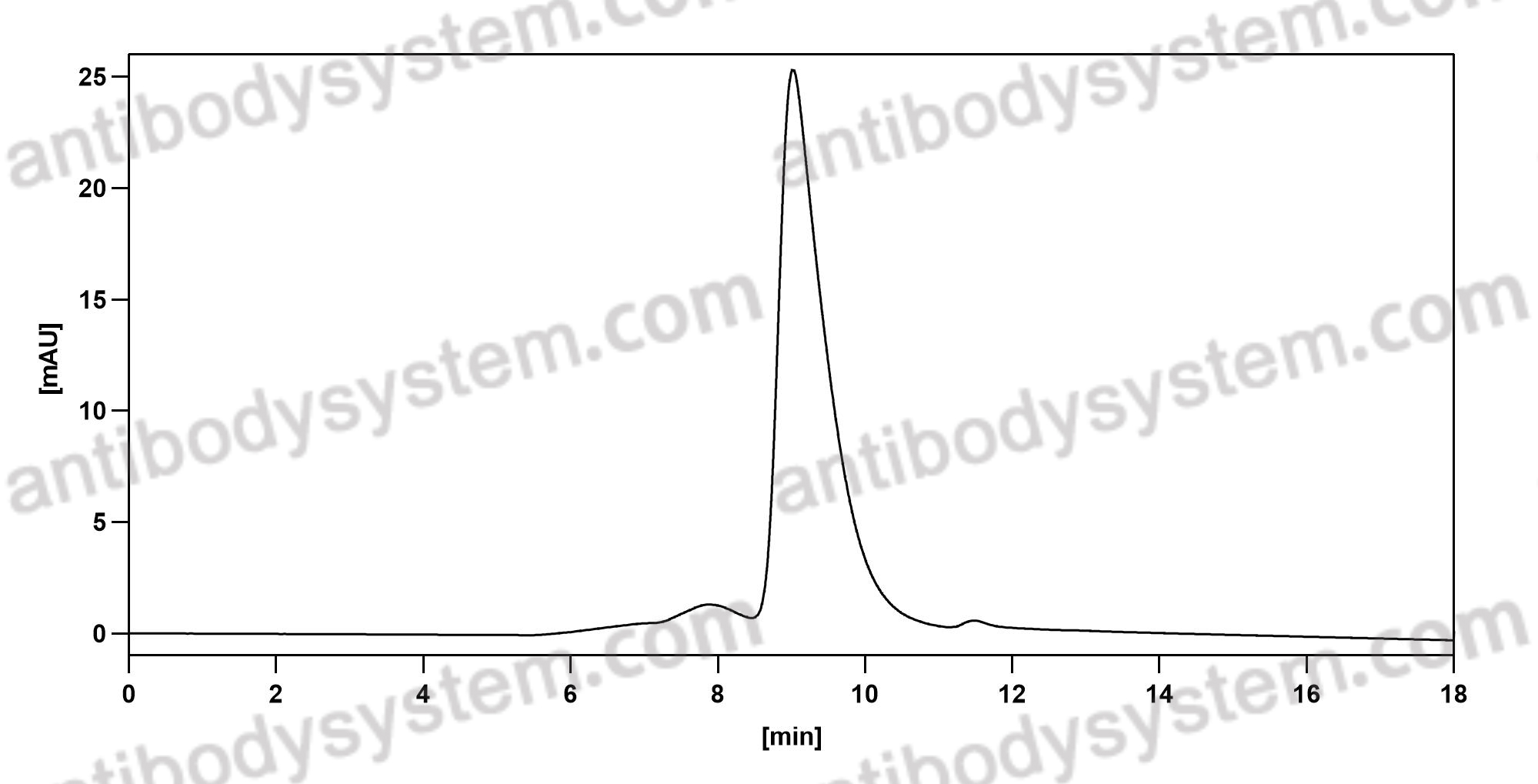
Purity
>90% as determined by SDS-PAGE.
Accession
Q9J3M8
Applications
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Form
Lyophilized
Storage buffer
Lyophilized from a solution in PBS pH 7.4, 5% Trehalose, 5% Mannitol.
Reconstitution
Reconstitute in sterile water for a stock solution.
Shipping
In general, proteins are provided as lyophilized powder/frozen liquid. They are shipped out with dry ice/blue ice unless customers require otherwise.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8°C for frequent use. Store at -20 to -80°C for twelve months from the date of receipt.
Alternative Names
Envelope glycoprotein E, gE, RF68
Immunogenicity and cellular response of a herpes zoster virus gEgI fusion protein adjuvanted with CpG-emulsion in mice., PMID:40448056
Varicella zoster virus mRNA vaccine candidate induced superior cellular immunity and comparable humoral and Fc-mediated immunity compared to the licensed subunit vaccine in a mouse model., PMID:40331755
A novel MF59 and CpG1018 adjuvant combination enhances the humoral and cellular immune responses against a truncated varicella-zoster viral glycoprotein E., PMID:40239819
Longitudinal single cell profiling of epitope specific memory CD4+ T cell responses to recombinant zoster vaccine., PMID:40057520
A simple, highly sensitive and specific serological test for the detection of antibodies to Varicella-zoster virus (Varicellovirus humanalpha3)., PMID:39841414
A VZV-gE subunit vaccine decorated with mPLA elicits protective cellular immmune responses against varicella-zoster virus., PMID:39799738
Truncated VZV gE Induces High-Titer Neutralizing Antibodies in Mice., PMID:39460306
Glycoprotein E-Displaying Nanoparticles Induce Robust Neutralizing Antibodies and T-Cell Response against Varicella Zoster Virus., PMID:39337359
Chimeric adenovirus-based herpes zoster vaccine with the tPA signal peptide elicits a robust T-cell immune response., PMID:39288613
The inactivated herpes zoster vaccine HZ/su induces a varicella zoster virus specific cellular and humoral immune response in patients on dialysis., PMID:39265505
Rational optimization of glycoprotein E (gE)-encoding mRNA for improved Varicella-zoster virus mRNA vaccine development., PMID:39137287
Heterologous Prime-Boost Immunization Strategies Using Varicella-Zoster Virus gE mRNA Vaccine and Adjuvanted Protein Subunit Vaccine Triggered Superior Cell Immune Response in Middle-Aged Mice., PMID:39130684
A nanoparticle vaccine displaying varicella-zoster virus gE antigen induces a superior cellular immune response than a licensed vaccine in mice and non-human primates., PMID:39081325
Varicella zoster virus-induced autophagy in human neuronal and hematopoietic cells exerts antiviral activity., PMID:38804180
Varicella zoster virus glycoprotein E facilitates PINK1/Parkin-mediated mitophagy to evade STING and MAVS-mediated antiviral innate immunity., PMID:38184594
Enhancing cell-scale performance via sustained release of the varicella-zoster virus antigen from a microneedle patch under simulated microgravity., PMID:38164004
Immunogenicity in Mice Immunized with Recombinant Adenoviruses Expressing Varicella-Zoster Virus Envelope Glycoprotein E., PMID:38140528
Rapid detection of varicella-zoster virus based on an immunochromatographic strip., PMID:37481958
Truncated glycoprotein E of varicella-zoster virus is an ideal immunogen for Escherichia coli-based vaccine design., PMID:36790656
Rapid, sensitive, and specific lateral-flow immunochromatographic point-of-care device for detection of varicella-zoster virus immunoglobulin G antibodies in fingerstick blood., PMID:36690067
Novel BC02 Compound Adjuvant Enhances Adaptive and Innate Immunity Induced by Recombinant Glycoprotein E of Varicella-Zoster Virus., PMID:36560565
LNP-CpG ODN-adjuvanted varicella-zoster virus glycoprotein E induced comparable levels of immunity with Shingrix™ in VZV-primed mice., PMID:35671982
Development of an Indirect ELISA Kit for Rapid Detection of Varicella-Zoster Virus Antibody by Glycoprotein E., PMID:35572642
An adjuvanted zoster vaccine elicits potent cellular immune responses in mice without QS21., PMID:35459225
Enhanced Potency and Persistence of Immunity to Varicella-Zoster Virus Glycoprotein E in Mice by Addition of a Novel BC02 Compound Adjuvant., PMID:35455278
Tofacitinib downregulates antiviral immune defence in keratinocytes and reduces T cell activation., PMID:34020693
Culture media optimization for Chinese hamster ovary cell growth and expression of recombinant varicella-zoster virus glycoprotein E., PMID:33897103
From virus to diabetes therapy: Characterization of a specific insulin-degrading enzyme inhibitor for diabetes treatment., PMID:33835493
Comparative Antibody Responses to the Live-Attenuated and Recombinant Herpes Zoster Vaccines., PMID:33762414
Evaluation of Recombinant Herpes Zoster Vaccine for Primary Immunization of Varicella-seronegative Transplant Recipients., PMID:33528118
CCR2- and Flt3-Dependent Inflammatory Conventional Type 2 Dendritic Cells Are Necessary for the Induction of Adaptive Immunity by the Human Vaccine Adjuvant System AS01., PMID:33519816
Short term results of vaccination with adjuvanted recombinant varicella zoster glycoprotein E during initial BTK inhibitor therapy for CLL or lymphoplasmacytic lymphoma., PMID:33128020
Immunogenicity generated by mRNA vaccine encoding VZV gE antigen is comparable to adjuvanted subunit vaccine and better than live attenuated vaccine in nonhuman primates., PMID:32703745
Exocytosis of Progeny Infectious Varicella-Zoster Virus Particles via a Mannose-6-Phosphate Receptor Pathway without Xenophagy following Secondary Envelopment., PMID:32493818
Comparison of a glycoprotein E-based ELISA with a varicella-zoster whole-virus ELISA for the quantification of varicella vaccine immune responses in young children., PMID:32184033
Evaluation of glycoprotein E subunit and live attenuated varicella-zoster virus vaccines formulated with a single-strand RNA-based adjuvant., PMID:32167678
Varicella-Zoster Virus infected human neurons are resistant to apoptosis., PMID:32125664
Recombinant zoster vaccine administration in an allergy and immunology practice: A medical and economic case., PMID:32088321
Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis., PMID:31399377
Understanding the immunology of Shingrix, a recombinant glycoprotein E adjuvanted herpes zoster vaccine., PMID:31003070
Humoral and cellular immunity against both ZIKV and poxvirus is elicited by a two-dose regimen using DNA and non-replicating vaccinia virus-based vaccine candidates., PMID:30851967
Efficient induction of cell-mediated immunity to varicella-zoster virus glycoprotein E co-lyophilized with a cationic liposome-based adjuvant in mice., PMID:30827737
Recombinant Glycoprotein E of Varicella Zoster Virus Contains Glycan-Peptide Motifs That Modulate B Cell Epitopes into Discrete Immunological Signatures., PMID:30813247
Binding varicella zoster virus: an underestimated facet of insulin-degrading enzyme´s implication for Alzheimer´s disease pathology?, PMID:30806771
Immune responses to zoster vaccines., PMID:30676834
Recombinant Zoster Vaccine (Shingrix®): A Review in Herpes Zoster., PMID:30370455
Expression of variable viruses as herpes simplex glycoprotein D and varicella zoster gE glycoprotein using a novel plasmid based expression system in insect cell., PMID:30294218
Cytokine Levels and Human Herpesviruses in Saliva from Clinical Periodontal Healthy Subjects with Peri-Implantitis: A Case-Control Study., PMID:30158834
Altered CD4+ T cell immunity in nurses occupationally exposed to viral pathogens., PMID:30076783
Complex interaction between mutant HNRNPA1 and gE of varicella zoster virus in pathogenesis of multiple sclerosis., PMID:29996671