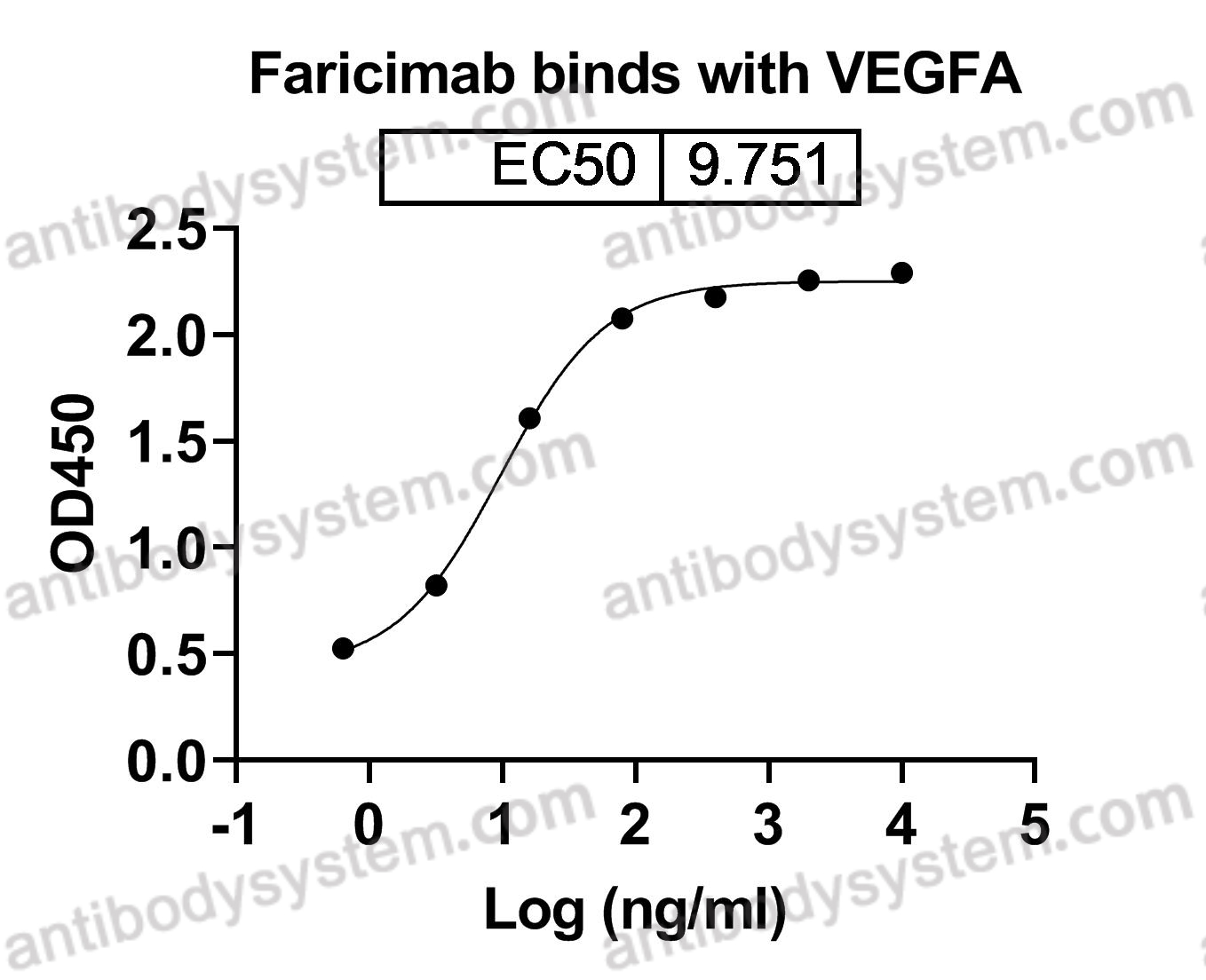
Simultaneous Inhibition of Angiopoietin-2 and Vascular Endothelial Growth Factor-A with Faricimab in Diabetic Macular Edema: BOULEVARD Phase 2 Randomized Trial, PMID: 30905643
Efficacy of Every Four Monthly and Quarterly Dosing of Faricimab vs Ranibizumab in Neovascular Age-Related Macular Degeneration: The STAIRWAY Phase 2 Randomized Clinical Trial, PMID: 32729897
Safety and Efficacy of Different Doses and Regimens of Faricimab vs Ranibizumab in Neovascular Age-Related Macular Degeneration: The AVENUE Phase 2 Randomized Clinical Trial, PMID: 32729888
Faricimab: an investigational agent targeting the Tie-2/angiopoietin pathway and VEGF-A for the treatment of retinal diseases, PMID: 33471572
Targeting Angiopoietin in Retinal Vascular Diseases: A Literature Review and Summary of Clinical Trials Involving Faricimab, PMID: 32785136
Faricimab: expanding horizon beyond VEGF, PMID: 31695160
Antibodies to watch in 2021, PMID: 33459118
Faricimab Combination Therapy for Sustained Efficacy in Neovascular Age-Related Macular Degeneration, PMID: 32729885
New therapeutic targets in the treatment of age-related macular degeneration, PMID: 31787390
Tie-2/Angiopoietin pathway modulation as a therapeutic strategy for retinal disease, PMID: 31513439
Emerging Therapies in Neovascular Age-Related Macular Degeneration in 2020, PMID: 32511123
Innovative therapies for neovascular age-related macular degeneration, PMID: 31298960
Emerging drugs for the treatment of diabetic retinopathy, PMID: 32715794
New Anti-VEGF Drugs in Ophthalmology, PMID: 32342814
The Tie2 signaling pathway in retinal vascular diseases: a novel therapeutic target in the eye, PMID: 33072401
Longer-acting treatments for neovascular age-related macular degeneration-present and future, PMID: 33432165
Wet age-related macular degeneration: treatment advances to reduce the injection burden, PMID: 32479026
Faricimab: Two in the Bush Is Proving Better than One in the Hand?, PMID: 34242102
Evolving treatment paradigms for PCV, PMID: 34262165
Next-generation anti-VEGF agents for diabetic macular oedema, PMID: 34373607
HIGHLIGHTS OF ADVANCES IN MEDICAL RETINA FROM THE VIRTUAL WORLD OPHTHALMOLOGY CONGRESS 2020, PMID: 33499644
Vascular Endothelial Growth Factor Antagonists: Promising Players in the Treatment of Neovascular Age-Related Macular Degeneration, PMID: 34188445
Development and evaluation of an ultrasensitive free VEGF-A immunoassay for analysis of human aqueous humor, PMID: 31070047
Molecular Features of Classic Retinal Drugs, Retinal Therapeutic Targets and Emerging Treatments, PMID: 34371793
Mechanisms of sterile inflammation after intravitreal injection of antiangiogenic drugs: a narrative review, PMID: 33962696
Faricimab Outcomes in Chorioretinal Disorders: Indian Real-World Analysis (FOCUS Study)., PMID:40529115
Faricimab for treatment-resistant choroidal neovascularization (CNV) in neovascular age-related macular degeneration (nAMD): seven-months results using artificial intelligence and OCTA., PMID:40528210
Intraocular inflammation following intravitreal injections of anti-vascular endothelial growth factor drugs., PMID:40522452
Quantification of Hyperreflective Foci in Age-related Macular Degeneration by Polarization-Sensitive OCT., PMID:40502292
Effect of Faricimab versus Aflibercept on Hyperreflective Foci in Patients with Diabetic Macular Edema from the YOSEMITE/RHINE Trials., PMID:40496217
Intraocular Inflammation Following Faricimab Intravitreal Injection Treated With Sub-tenon Triamcinolone Acetonide Injection., PMID:40491639
Anti-VEGFs for Diabetic Macular Oedema: Analysis of Efficacy, Safety, and Cost of More Durable Therapies from a Dutch Societal Perspective., PMID:40481910
Retinal Vasculitis after Intravitreal Faricimab Injection., PMID:40478546
Early Anatomical and Functional Outcomes of Faricimab in Recalcitrant Neovascular Age-Related Macular Degeneration: A Retrospective Real-World Study in an East-Asian Population., PMID:40465138
Author Correction: Effects of switching from intravitreal injection of aflibercept to faricimab on ocular blood flow in patients with diabetic macular edema., PMID:40456888
Association between intravitreal anti-vascular endothelial growth factor agents and hypertension: a meta-analysis., PMID:40456283
Real-Life Treatment Intervals and Morphological Outcomes Following the Switch to Faricimab Therapy in Neovascular Age-Related Macular Degeneration., PMID:40423060
Effectiveness and safety of anti-vascular endothelial growth factor therapies for macular edema in retinal vein occlusion: A systematic review and network meta-analysis of randomized controlled trials., PMID:40419166
Successful Response to Intravitreal Faricimab Injections in a Case of Neovascular Age-Related Macular Degeneration in Vitrectomized Eyes., PMID:40400877
[Faricimab in previously treated neovascular age-related macular degeneration : Study design of the prospective noninterventional study PASSENGER]., PMID:40397174
Twelve-Month Outcomes of Faricimab for Patients with Sub-Optimally Responsive Diabetic Macular Oedema: A Retrospective Single-Centre Study., PMID:40396158
Early Outcomes After Initiation of Faricimab in Patients With Diabetic Macular Edema., PMID:40371971
Early Outcomes After Initiation of Faricimab for Neovascular Age-Related Macular Degeneration., PMID:40371970
Faricimab Reverts VEGF-A165-Induced Impairment of the Barrier Formed by Retinal Endothelial Cells., PMID:40362554
Silicone oil microdroplets from syringes with intravitreal anti-vascular endothelial growth factor and complement inhibitor injections., PMID:40359347
Short-term real-world outcomes of diabetic macular edema treated with intravitreal faricimab., PMID:40359224
Real-World Data on Morphological and Functional Responses After Switching to Faricimab in Recalcitrant, Chronic Diabetic Macular Edema., PMID:40355730
Outcomes by Faricimab Treatment Interval at Week 48 of TENAYA-LUCERNE Phase 3 Trials in Neovascular Age-Related Macular Degeneration., PMID:40349982
Two-year outcomes of treat-and-extend regimen with intravitreal faricimab for treatment-naïve neovascular age-related macular degeneration., PMID:40347367
Improved functional and morphological outcomes with faricimab in nAMD eyes with poor response to prior intravitreal anti-VEGF therapy., PMID:40338383
One-year functional and structural results of faricimab for treatment-naïve neovascular age related macular degeneration: An OCT angiography study., PMID:40329091
Tailored Anti-VEGF Therapy with New Generation Optimizations (TANGO) Treatment Regimen for Neovascular Age-Related Macular Degeneration: Rationale, Design, and Simulation Study., PMID:40321164
Short-term comparison of switching to faricimab from other anti-VEGF agents in neovascular age-related macular degeneration patients: A retrospective study., PMID:40295235
Intraocular Inflammation, Safety Events, and Outcomes After Intravitreal Injection of Ranibizumab, Aflibercept, Brolucizumab, Abicipar Pegol, and Faricimab for nAMD., PMID:40271423
Bilateral Sterile Granulomatous Uveitis Caused by Intravitreal Injections of Faricimab., PMID:40271422
[Intravitreal Faricimab Injections for Coats Disease: a Case Report]., PMID:40267947
Early Outcomes of Faricimab Treatment for Diabetic Macular Edema in Patients Previously Treated With Anti-VEGF Therapy., PMID:40258191
Real-World Evidence for Faricimab in Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema: A Scoping Review., PMID:40242371
Comparative efficacy of intravitreal anti-VEGF therapy for neovascular age-related macular degeneration: A systematic review with network meta-analysis., PMID:40241463
Intravitreal injection of faricimab to treat macular and retinovascular diseases in Nigerians: Early real-world experience., PMID:40233664
Safety profile of faricimab: a multi-source pharmacovigilance analysis using FAERS and JADER., PMID:40221797
Switching to Faricimab in Therapy-Resistant Macular Edema Due to Retinal Vein Occlusion: Initial Real-World Efficacy Outcomes., PMID:40217902
Deep learning assisted analysis of biomarker changes in refractory neovascular AMD after switch to faricimab., PMID:40217505
Efficacy of Aflibercept (8 mg) for Diabetic Macular Edema in Vitrectomized Eyes Refractory to the Other Anti-VEGF Drug Therapies: A Report of Three Cases., PMID:40206271
Occlusive Retinal Vasculitis After Aflibercept 8mg Injection for Wet Macular Degeneration., PMID:40184546
Adverse event reporting of faricimab: a disproportionality analysis of FDA adverse event reporting system (FAERS) database., PMID:40144657
One-Year Outcomes after Switching to Faricimab in Eyes with Pretreated Neovascular Age-Related Macular Degeneration: A Swiss Retina Research Network Report., PMID:40139459
Efficacy of treatment with faricimab for patients with refractory nAMD., PMID:40134284
Deep-Learning-Assisted Analysis of Early Biomarker Changes in Treatment-Naïve Patients with Neovascular AMD Under Intravitreal Faricimab., PMID:40133689
Surprising complete regression of a choroidal neovascular membrane with faricimab., PMID:40120750
Faricimab Treat-and-Extend Dosing for Macular Edema Due to Retinal Vein Occlusion: 72-Week Results from the BALATON and COMINO Trials., PMID:40107501
Recurrent uveitic macular edema managed with intravitreal faricimab injection., PMID:40080237
Cost-effectiveness analysis of bispecific antibody faricimab for treatment of neovascular age-related macular degeneration and diabetic macular edema in Japan., PMID:40078048
Comparison of intraocular pressure changes in Japanese patients with neovascular age-related macular degeneration treated with aflibercept or faricimab., PMID:40072815
Economic impact of a faricimab vial-sharing protocol in age-related macular degeneration and diabetic macular oedema patients., PMID:40071859