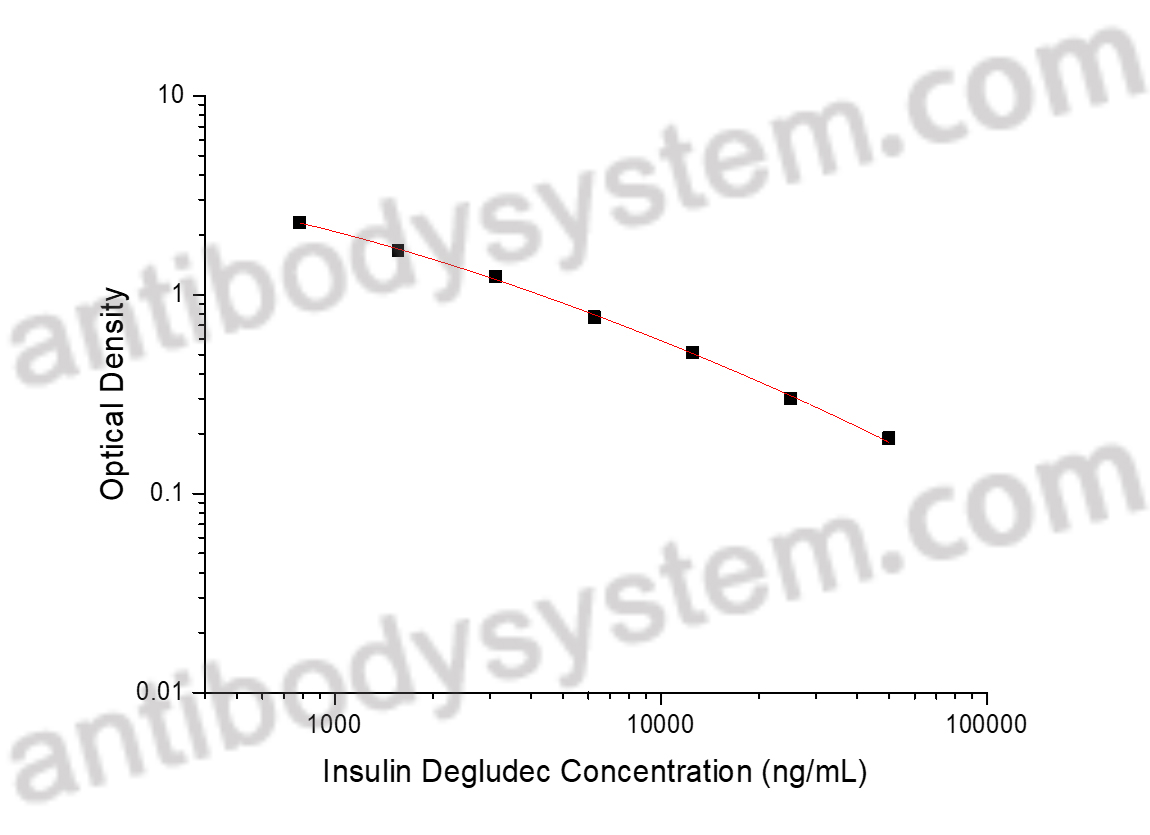
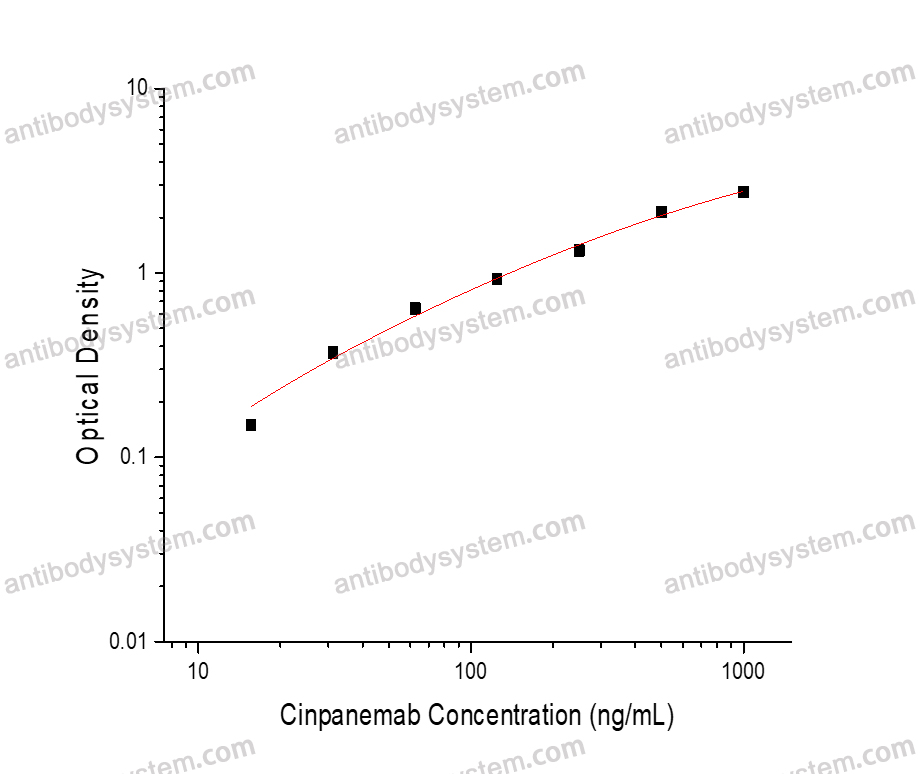
Evaluation of an Alternative Approach to Managing Diabetic Ketoacidosis: Combination Rapid-Acting and Basal Subcutaneous Insulin (CRABI-DKA)., PMID:40390456
Structural analysis of a micron-sized deposit of Cu0 in an insulin ball from a person with diabetes., PMID:40328936
Investigation of new non-toxic inhibitors of fibril formation and preservatives for insulin preparations its analogues., PMID:40324845
Diffusion dimensionality modeling of subcutaneous/intramuscular absorption of antibodies and long-acting injectables., PMID:40263185
Reduction of Postprandial Glucose Excursions in Adults, Adolescents, and Children with Type 1 Diabetes Using Ultra-Rapid Lispro Insulin and Control-IQ+ Technology., PMID:40171722
Pharmacokinetics and pharmacodynamics of insulin lispro 25 versus the original preparation (Humalog®25) in Chinese healthy male volunteers., PMID:40070573
What do the guidelines say about use of biosimilar insulin therapy? Simple practical considerations to guide clinicians in different patient subgroups-Sharing Canadian perspectives., PMID:40013436
A comparison of ultra-rapid and rapid insulin in automated insulin delivery for type 1 diabetes: A systematic review and meta-analysis of randomized controlled trials., PMID:39996365
Comparing the Efficacy of Various Insulin Types: Pharmacokinetic and Pharmacodynamic Modeling of Glucose Clamp Effects in Healthy Volunteers., PMID:39982761
Switching to insulin lispro U-200 in a pregnant woman using a 780G advanced hybrid closed-loop led to a rapid improvement in glucose metrics., PMID:39961514
Efforts of the Pharmaceuticals and Medical Devices Agency of Japanese regulatory agency in supporting biosimilar development and disseminate information., PMID:39951116
First Dimension Trap-and-Elute Combined with Second Dimension Reversed-Phase Liquid Chromatography Separation Using a Two-Dimensional-Liquid Chromatography-Tandem Mass Spectrometry System for Sensitive Quantification of Human Insulin and Six Insulin Analogs in Plasma: Improved Chromatographic Resolution and Stability Testing., PMID:39930590
Efficacy and hypoglycaemia outcomes with once-weekly insulin icodec versus once-daily basal insulin in individuals with type 2 diabetes by kidney function: A post hoc participant-level analysis of the ONWARDS 1-5 trials., PMID:39930546
Cost-Effectiveness Analysis of Pharmacological Treatment With Insulin and Insulin Analogs for Type 1 and Type 2 Diabetes Mellitus in Colombia., PMID:39919670
The insight into the intermolecular interactions between protamine and insulin lispro., PMID:39889553
Pharmacotherapy of type 1 diabetes - part 1: yesterday., PMID:39875200
Pharmacokinetic and Pharmacodynamic Equivalence of Biosimilar and Reference Ultra-Rapid Lispro: A Comparative Clamp Study in Healthy Volunteers., PMID:39778084
Real world pharmacovigilance assessment of drug related macular degeneration risks., PMID:39774257
Regular human insulins versus rapid-acting insulin analogues in children and adolescents with type 1 diabetes: a protocol for a systematic review with meta-analysis and Trial Sequential Analysis., PMID:39763007
Anxiety and Body Image Distress in a Type 1 Diabetes Patient With Insulin-Induced Lipodystrophy., PMID:39759721
Newer Insulin Preparations and Insulin Analogs., PMID:39734941
[Tirzepatide : overview of clinical studies SURPASS in type 2 diabetes and SURMOUNT in obesity]., PMID:39697128
Role of the liver in the sustained normalisation of A1c over 2 years following short-term insulin therapy in early type 2 diabetes., PMID:39609927
Investigation of Severe Hypoglycemia Risk Among Patients with Diabetes Treated with Ultra-Rapid Lispro in Japan., PMID:39570544
Safety Profiles Related to Dosing Errors of Rapid-Acting Insulin Analogs: A Comparative Analysis Using the EudraVigilance Database., PMID:39457586
Impact of Ultra-Rapid Insulin on Boost and Ease-Off in the Cambridge Hybrid Closed-Loop System for Individuals With Type 1 Diabetes., PMID:39422158
Anaphylactic Reaction to Protamine Sulfate: A Case Report., PMID:39421103
A Glycemic Threshold Above Which the Improvement of β-Cell Function and Glycemia in Response to Insulin Therapy Is Amplified in Early Type 2 Diabetes: The Reversal of Glucotoxicity., PMID:39302842
Once-weekly insulin efsitora alfa versus once-daily insulin degludec in adults with type 1 diabetes (QWINT-5): a phase 3 randomised non-inferiority trial., PMID:39270686
Sub-massive Pulmonary Embolism and New-Onset Diabetes Mellitus., PMID:39156451
Optimization of a mass spectrometric analytical method for the quality assessment of insulin and its analogs., PMID:39152556
Higher Order Structure Differences Among Insulin Crystalline Drugs Revealed by 2D heteronuclear NMR., PMID:39116305
ADO09, a co-formulation of pramlintide and insulin A21G, lowers body weight versus insulin lispro in type 1 diabetes., PMID:39109464
Pregnancy-associated fulminant type 1 diabetes: a case report and review of the literature., PMID:39101176
Tirzepatide Improved Health-Related Quality of Life Compared with Insulin Lispro in Basal Insulin-Treated Adults with Type 2 Diabetes and Inadequate Glycaemic Control: A Randomised Controlled Phase 3b Trial (SURPASS-6)., PMID:39008236
Comparison of the efficacy and safety of rapid-acting insulin analogs, lispro versus aspart, in the treatment of diabetes: a systematic review of randomized controlled trials., PMID:38934226
Ultra rapid lispro improves postprandial glucose control versus lispro in combination with basal insulin: a study based on CGM in type 2 diabetes in China., PMID:38774225
Therapy-Related Satisfaction and Quality of Life for Japanese People with Diabetes Using Rapid-Acting Insulin Analogs: A Web-Based Survey., PMID:38760595
4S-fluorination of ProB29 in insulin lispro slows fibril formation., PMID:38703998
Multicenter Evaluation of Ultra-Rapid Lispro Insulin with Control-IQ Technology in Adults, Adolescents, and Children with Type 1 Diabetes., PMID:38696672
Efficacy and safety of basal insulins in people with type 2 diabetes mellitus: a systematic review and network meta-analysis of randomized clinical trials., PMID:38586456
Prandial Insulins: A Person-Centered Choice., PMID:38568467
Are New Ultra-Rapid-Acting Insulins Associated with Improved Glycemic Control and Reduced Hypoglycemia in Comparison to Conventional Rapid-Acting Insulins for Individuals with Type 1 and Type 2 Diabetes? A Systematic Review and Meta-Analysis., PMID:38502158
Biorelevant subcutaneous in vitro test predicts the release of human and fast acting insulin formulations., PMID:38490402
Is it really possible to kill with insulin without leaving traces? From lifesaver to killer, the issues surrounding the analytical characterization of postmortem insulin illustrated by an exemplary case., PMID:38481368
Efficacy and Safety of Ultra-rapid Lispro Insulin in Managing Type-1 and Type-2 Diabetes: A Systematic Review and Meta-Analysis., PMID:38371177
Postprandial Glucose Excursions with Ultra-Rapid Insulin Analogs in Hybrid Closed-Loop Therapy for Adults with Type 1 Diabetes., PMID:38315506