Catalog No.
KDE22001
Description
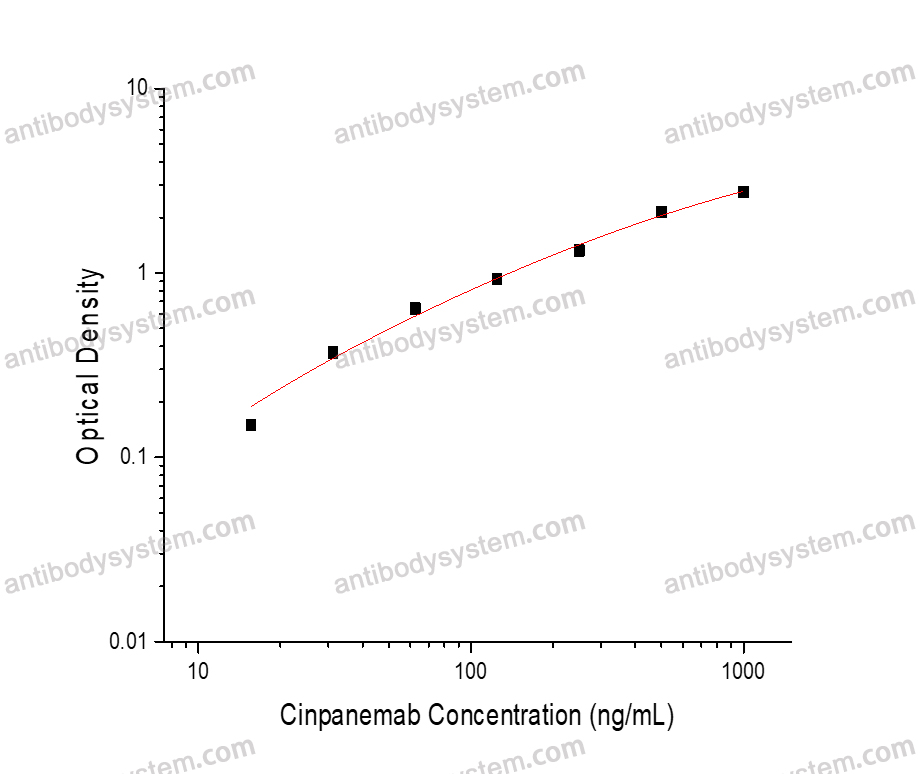
PRINCIPLE OF THE ASSAY This assay employs the quantitative indirect enzyme immunoassay technique. Human SNCA has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Cinpanemab present is bound by the immobilized protein. After washing away any unbound substances, a biotin-labeled Mouse Anti-Human IgG antibody is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Cinpanemab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Cinpanemab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
15.63 - 1,000 ng/mL
Sensitivity
4.25 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision |
Inter-Assay Precision |
||||
|
Sample |
1 |
2 |
3 |
1 |
2 |
3 |
|
n |
16 |
16 |
16 |
24 |
24 |
24 |
|
Mean (ng/mL) |
535.7 |
124.2 |
20.1 |
499.0 |
98.0 |
15.1 |
|
Standard deviation |
41.5 |
11.0 |
2.0 |
52.9 |
12.0 |
2.0 |
|
CV (%) |
7.8 |
8.8 |
9.9 |
10.6 |
12.2 |
13.4 |
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
BIIB-054, CAS: 2094516-02-4
Background
Cinpanemab is a human-derived monoclonal antibody directed against α-synuclein. Genetic and pathology evidence implicate this protein in the molecular pathogenesis of Parkinson's disease (PD) and other α-synucleinopathies such as dementia with Lewy bodies (DLB). In 2010, Biogen licensed cinpanemab from Neurimmune. The antibody was generated with reverse translational medicine technology from naturally occurring, presumably protective antibodies found in healthy aged donors, as were aducanumab and other antibodies in the Biogen-Neurimmune partnership. The antibody, previously known as BIIB054, binds to α-synuclein residues 1-10, with 800-fold higher affinity for aggregated over monomeric α-synuclein. The antibody inhibits α-synuclein spreading in cell-based assays, and slows pathology and motor symptoms in mice (Weihofen et al., 2019).