Catalog No.
KAJ63101
Description
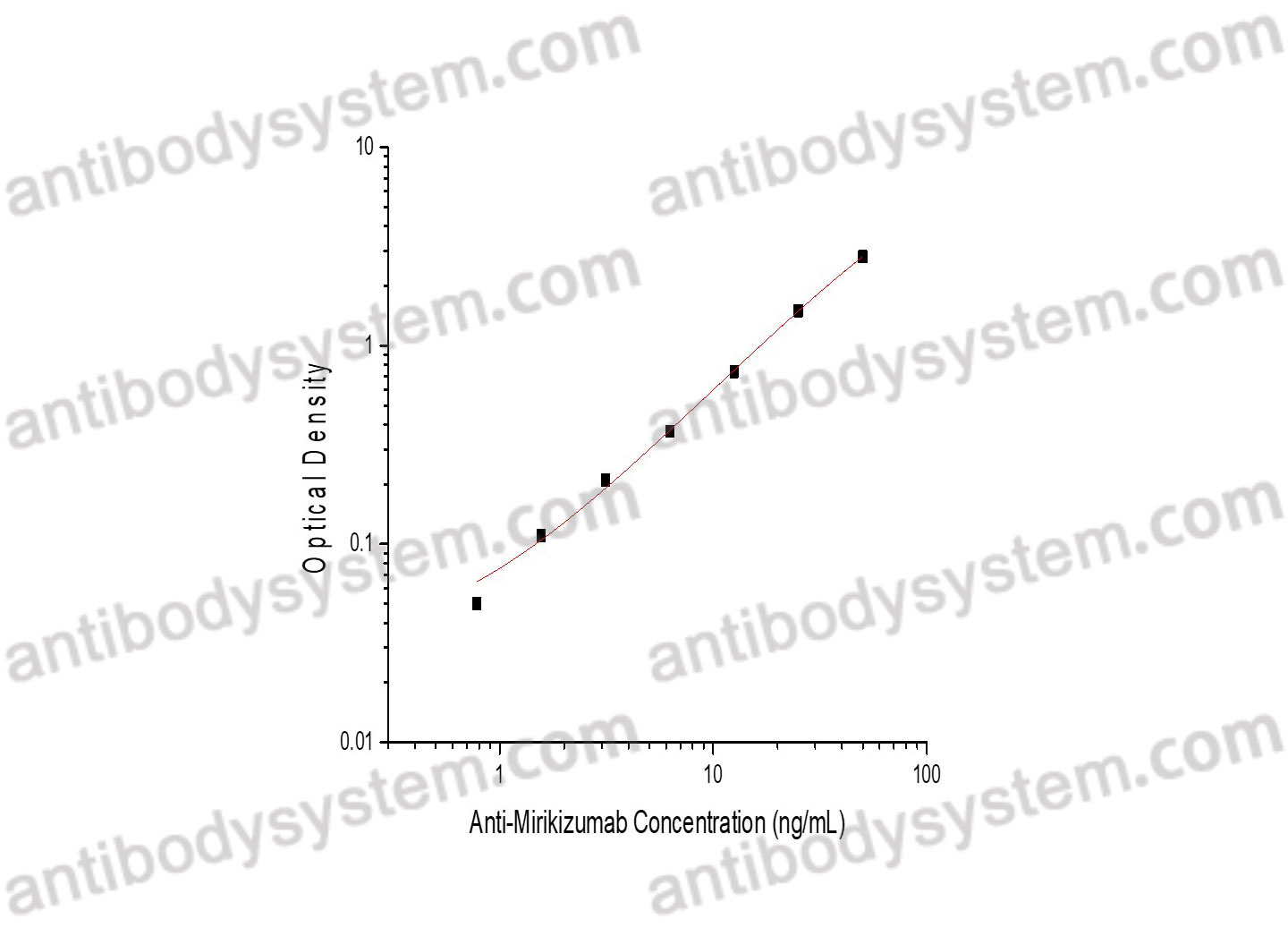
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Mirikizumab has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and Anti-Mirikizumab will be captured by immobilized Mirikizumab. After washing away any unbound substances, a biotin-labeled Mirikizumab is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Mirikizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Mirikizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
0.78 - 50 ng/mL
Sensitivity
0.43 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
22.7
|
6.2
|
1.4
|
25.3
|
6.5
|
1.3
|
|
Standard deviation
|
1.0
|
0.3
|
0.1
|
1.5
|
0.5
|
0.2
|
|
CV (%)
|
4.3
|
4.7
|
6.9
|
6.0
|
7.8
|
14.4
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
LY-3074828, CAS: 1884201-71-1
Impact of Mirikizumab Treatment on Fatigue in Patients with Moderately to Severely Active Crohn's Disease: Results from the Phase 3 VIVID-1 Study., PMID:40491246
Interleukin-23 Inhibitors for Inflammatory Bowel Disease: Pivotal Trials and Practical Considerations., PMID:40465057
Guselkumab (Tremfya) - an IL-23 antagonist for Crohn's disease., PMID:40459404
IL-17A inhibitor-induced ulcerative colitis treated with an anti-IL-23 antibody., PMID:40455166
First Documented Case of Successful Dual Therapy With Upadacitinib and Mirikizumab for Multi-Refractory Ulcerative Proctitis., PMID:40411442
With guselkumab, does the dual mechanisms to inhibit IL-23, help in ulcerative colitis?, PMID:40394835
Cost per remission for mirikizumab versus ustekinumab for moderately to severely active ulcerative colitis treatment from the United States commercial payer perspective., PMID:40351121
Mirikizumab (Omvoh) - an IL-23 antagonist for Crohn's disease., PMID:40324965
Editorial: Real-World Effectiveness of Mirikizumab in Ulcerative Colitis-A Valuable but Preliminary Glimpse. Authors' Reply., PMID:40321062
Editorial: Real-World Effectiveness of Mirikizumab in Ulcerative Colitis-A Valuable but Preliminary Glimpse., PMID:40321049
Efficacy and safety of Mirikizumab for ulcerative colitis: a systematic review and meta-analysis of randomized controlled trials., PMID:40301737
Efficacy and safety of mirikizumab in the treatment of inflammatory bowel disease: A meta-analysis., PMID:40295305
New Interleukin-23 Antagonists' Use in Crohn's Disease., PMID:40283885
Efficacy and Safety of Mirikizumab in the Treatment of Moderately to Severely Active Ulcerative Colitis Regardless of Baseline Modified Mayo Score: Results From the Phase 3 LUCENT Trials., PMID:40260308
Mirikizumab for Ulcerative Colitis: A Game-Changer or Just Another Incremental Advance?, PMID:40237908
Real-World Effectiveness and Safety of Mirikizumab Induction Therapy in Patients With Ulcerative Colitis: A Multicentre Retrospective Observational Study., PMID:40205711
Efficacy of Mirikizumab in Patients with Prior Ustekinumab Exposure: A Case Series., PMID:40198002
Pharmacokinetic Comparability and Safety Between Original and Citrate-Free Mirikizumab Formulations for Subcutaneous Injections: Results from Three Clinical Trials., PMID:40117091
Paradigm Shift in Inflammatory Bowel Disease Management: Precision Medicine, Artificial Intelligence, and Emerging Therapies., PMID:40095460
IL23p19 therapies for moderately-to-severely active ulcerative colitis., PMID:40082083
Mirikizumab Improves Quality of Life and Work Productivity in Patients with Moderately to Severely Active Crohn's Disease: Results from the Phase 3 VIVID-1 Study., PMID:40079470
Long-Term Safety of Mirikizumab in Patients With Moderately to Severely Active Ulcerative Colitis: An Integrated 2-Year Safety Analysis., PMID:40071779
Combining mechanistic modeling with machine learning as a strategy to predict inflammatory bowel disease clinical scores., PMID:40070575
Guselkumab (Tremfya) for ulcerative colitis., PMID:40053377
Mirikizumab in the Treatment of Ulcerative Colitis: Initial Real-World Data in a Population from a Large Tertiary Center., PMID:40048134
Drug Development in Inflammatory Bowel Diseases: What Is Next?, PMID:40006003
Epidemiology, pathogenesis, diagnosis, and treatment of inflammatory bowel disease: Insights from the past two years., PMID:39994836
The VIVID-1 study: A novel methodological approach provides further evidence of efficacy of selective IL-23 inhibition in Crohn's disease., PMID:39954670
Efficacy and safety of mirikizumab in psoriasis: a systematic review and meta-analysis of randomized controlled trials., PMID:39954188
Role of Mirikizumab in the Treatment of Inflammatory Bowel Disease-From Bench to Bedside., PMID:39941671
Mirikizumab for Crohn's Disease: Small Step Forward or Giant Leap Ahead?, PMID:39914777
Long-Term Endoscopic and Histological Outcomes of Mirikizumab in Patients With Moderately to Severely Active Ulcerative Colitis With Up to 3 Years of Treatment., PMID:39896691
Exit Interviews Exploring Patients' Experience of Change in Crohn's Disease Symptoms During the Mirikizumab Phase 3 Clinical Trial In Adult Patients With Moderately-to-Severely Crohn's Disease., PMID:39834356
Mirikizumab pharmacokinetics and exposure-response in pediatric patients with moderate-to-severe ulcerative colitis., PMID:39693227
The Urgency Numeric Rating Scale: Psychometric Evaluation in Adults with Crohn's Disease., PMID:39692838
Targeting mucosal healing in Crohn's disease: efficacy of novel pathways and therapeutic targets., PMID:39611536
Efficacy and safety of mirikizumab in patients with moderately-to-severely active Crohn's disease: a phase 3, multicentre, randomised, double-blind, placebo-controlled and active-controlled, treat-through study., PMID:39581202
AGA Living Clinical Practice Guideline on Pharmacological Management of Moderate-to-Severe Ulcerative Colitis., PMID:39572132
Mirikizumab for Chronic Plaque Psoriasis: A Systematic Review and Meta-Analysis., PMID:39570737
Risankizumab (Skyrizi) for ulcerative colitis., PMID:39509158
Patient and Healthcare Professional Satisfaction, Acceptability, and Preference Experiences With Mirikizumab Administration for Ulcerative Colitis: An International Survey., PMID:39473630
Three-Year Efficacy and Safety of Mirikizumab Following 152 Weeks of Continuous Treatment for Ulcerative Colitis: Results From the LUCENT-3 Open-Label Extension Study., PMID:39448057
Comparative Efficacy of Advanced Therapies for Management of Moderate-to-Severe Ulcerative Colitis: 2024 American Gastroenterological Association Evidence Synthesis., PMID:39425738
Efficacy and Safety of Advanced Therapies in Moderately-to-Severely Active Ulcerative Colitis: a Systematic Review and Network Meta-analysis., PMID:39404996
Network Meta-Analysis: Histologic and Histo-Endoscopic Improvement and Remission With Advanced Therapy in Ulcerative Colitis., PMID:39367678
An evaluation of mirikizumab for the treatment of ulcerative colitis., PMID:39360778
Mirikizumab for the treatment of chronic antibiotic-refractory pouchitis., PMID:39321965
Efficacy and safety of mirikizumab compared with currently approved biologic drugs for the treatment of ulcerative colitis: A systematic review and network meta-analysis., PMID:39320112
Recent clinical evidence on nutrition, novel pharmacotherapy, and vaccination in inflammatory bowel diseases., PMID:39308999
Safety of Rotavirus Vaccination in Infants That Were Exposed to Biologics In Utero: A Systematic Review., PMID:39303214