Catalog No.
KDD12601
Description
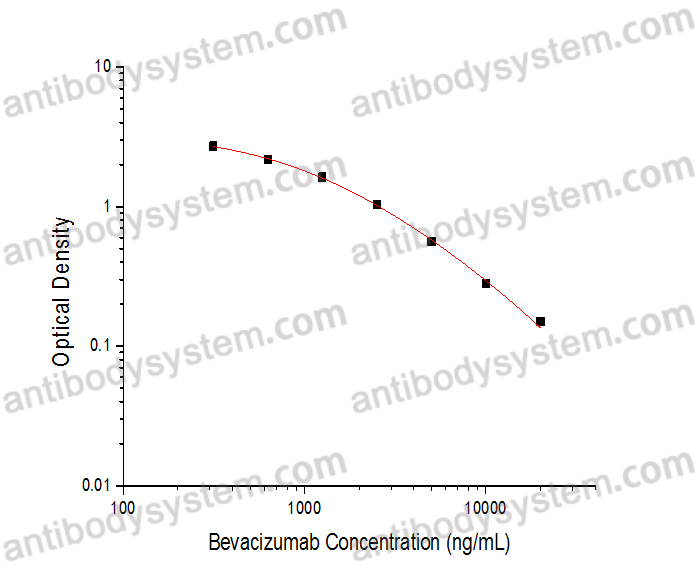
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human VEGFA has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Bevacizumab in the sample competitively binds to the pre-coated protein with biotin-labeled Bevacizumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Bevacizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Bevacizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
312.5 - 20,000 ng/mL
Sensitivity
98.1 ng/mL
Precision
PRECISION
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
11639.2
|
2962.4
|
724.5
|
11135.5
|
2820.1
|
722.6
|
|
Standard deviation
|
908.9
|
136.1
|
61.5
|
884.1
|
143.4
|
73.1
|
|
CV (%)
|
7.8
|
4.6
|
8.5
|
7.9
|
5.1
|
10.1
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20℃, the rest reagents should be store at 4℃.
Alternative Names
12-IgG1, F(ab)-12 IgG1, Fab-12 IgG1, rhuMAb-VEGF, ABP 215, CAS: 216974-75-3
Background
Bevacizumab is a recombinant human IgG1 monoclonal antibody specific for all human vascular endothelial growth factor-A (VEGF-A) isoforms. The humanized anti-VEGF monoclonal antibody, bevacizumab, has been approved by the FDA as a first-line treatment for metastatic colorectal cancer in combination with chemotherapy. The pharmacokinetic properties of bevacizumab in several species have been previously described and are consistent with a typical humanized monoclonal antibody. It was shown in the literature that the surveillance of circulating concentration during maintenance therapy represents a direct and/or indirect factor for some other side effects. Identification of biomarkers for (non-)response and risk factors for adverse drug reactions that might be related to serum concentrations and maintaining the effective concentration of Bevacizumab in order to potentially avoid some side effects with a reliable method might be beneficial.
Bevacizumab-Conjugated Curcumin Nanoparticles Promote Cytotoxicity and Apoptosis in Human Malignant Oral Keratinocytes In Vitro., PMID:40514017
Predictive value of BRCA1/RAD51C methylation in HGSOC - An ancillary study of the PAOLA-1/ENGOT-ov25 phase 3 trial., PMID:40513287
Increased Hemorrhage During Excision of Bevacizumab-Treated NF2-Related Vestibular Schwannomas., PMID:40511882
Advances in Molecular Imaging of VEGFRs: Innovations in Imaging and Therapeutics., PMID:40508182
Real-World Experiences Using Atezolizumab + Bevacizumab for the Treatment of Unresectable Hepatocellular Carcinoma: A Multicenter Study., PMID:40507295
Revised European Society of Endocrinology Clinical Practice Guideline for the management of aggressive pituitary tumours and pituitary carcinomas., PMID:40506054
Advances in drug treatments for male patients with prolactinomas., PMID:40505642
The use of Bevacizumab in the treatment of brain arteriovenous malformations: a systematic review., PMID:40504282
Clinical and prognostic characteristics of metastatic colorectal cancer with minor RAS mutations., PMID:40503036
Mirvetuximab soravtansine for the treatment of epithelial ovarian, fallopian tube, or primary peritoneal cancer., PMID:40501444
A Neovascularization and EGFR Dual-Targeting Gold Nanotheranostic System for Non-Small Cell Lung Cancer Treatment., PMID:40500941
Response to the comment on our manuscript "Effect of systemic FOLFOXIRI plus bevacizumab treatment of colorectal peritoneal metastasis on local and systemic immune cells"., PMID:40500641
Targeting the ST3 beta-galactoside alpha-2,3-sialyltransferase 1 (ST3Gal1) as a potential therapeutic strategy to overcome anti-VEGF resistance in endometrial cancer., PMID:40497576
Obesity paradox role in the immunosuppressive treatment of hepatocellular carcinoma., PMID:40497086
Adoptive cellular immunotherapy combined with chemotherapy versus chemotherapy alone in Chinese patients with metastatic colorectal cancer: a cost-effectiveness analysis to inform drug pricing., PMID:40496606
Bevacizumab in recurrent epithelial ovarian cancer: real-world experience from a tertiary cancer hospital in India., PMID:40496305
Antineovascular Effects of Coenzyme Q10 and Vitamin E Combination in Corneal Neovascularization: A Pilot Study., PMID:40495727
Atezolizumab plus bevacizumab versus Lenvatinib for patients with Barcelona clinic liver cancer stage B (BCLC-B) hepatocellular carcinoma (HCC): A real-world population., PMID:40494131
Whole Body-MRI assessment of peripheral lesions in patients with NF2-related schwannomatosis on systemic bevacizumab., PMID:40493293
Children with medulloblastoma treated with modified ACNS0821 temozolomide, irinotecan, and bevacizumab: The Seattle Children's Hospital experience., PMID:40487589
Lenvatinib versus bevacizumab when combined with PD-1/L1 inhibitor and hepatic arterial infusion chemotherapy in unresectable hepatocellular carcinoma., PMID:40486515
Response to 'Comment on: 'Comparing safety and efficacy of Bevacizumab, Ranibizumab and Ranibizumab biosimilar in Retinopathy of prematurity"., PMID:40483306
Personalized bevacizumab dosing in metastatic colorectal cancer: Statistical considerations for a phase III randomized trial., PMID:40483175
Multimodal nanoparticles co-delivering bevacizumab and dichloroacetate for dual targeting of neoangiogenesis and hyperglycolysis in glioblastoma treatment., PMID:40482922
Revisiting the Role of Intravitreal Triamcinolone in Diabetic Macular Edema: 12-Month Outcomes after Bevacizumab Failure., PMID:40481980
Anti-VEGFs for Diabetic Macular Oedema: Analysis of Efficacy, Safety, and Cost of More Durable Therapies from a Dutch Societal Perspective., PMID:40481910
Characteristics and outcomes of primary and secondary resistance to immune checkpoint inhibitors in hepatocellular carcinoma., PMID:40481877
Intravitreal anti-vascular endothelial growth factor injections and risks of stroke in patients with neovascular age-related macular degeneration-A registry-based cohort study., PMID:40481786
Efficacy of Hepatic Artery Infusion Chemotherapy with Bevacizumab and Sintilimab in Advanced Hepatocellular Carcinoma: A Case Report., PMID:40481624
Safety and efficacy of systemic chemotherapy plus PD-1 inhibitor in combination with intravenous or intraperitoneal bevacizumab in gastric cancer with peritoneal metastasis., PMID:40481442
Validation of a 15-Gene Prognostic Signature in Metastatic Clear Cell Renal Cell Carcinoma., PMID:40479621
A case report of small cell neuroendocrine carcinoma of the ovary and review of the literature., PMID:40475766
Bevacizumab in ovarian cancer therapy: current advances, clinical challenges, and emerging strategies., PMID:40474872
Relacorilant and nab-paclitaxel in patients with platinum-resistant ovarian cancer (ROSELLA): an open-label, randomised, controlled, phase 3 trial., PMID:40473448
Clinical characteristics and treatment response of treatment requiring retinopathy of prematurity (ROP) in Big Premature Infants in Turkiye: BIG-ROP Study Group Report No 2 (BIG-ROP STUDY)., PMID:40473274
Transarterial chemoembolization combined with intra-arterial infusion of sintilimab and bevacizumab for advanced hepatocellular carcinoma: a phase 2 study., PMID:40472923
Design and characterization of intravitreal bevacizumab-loaded PLGA nanoparticles: pharmacokinetic and biodistribution impact., PMID:40471504
Complete Response of Hepatocellular Carcinoma With Inferior Vena Cava Tumor Thrombus and Right Atrium Involvement to Combined Radiotherapy and Immunotherapy: A Case Report., PMID:40470485
Refractory Diarrhea Related to EPHB4 Mutation in a Patient With Capillary Malformation-Arteriovenous Type 2 Syndrome., PMID:40469463
PD-1/PD-L1 inhibitors plus bevacizumab plus chemotherapy versus PD-1/PD-L1 inhibitors plus chemotherapy for advanced non-small cell lung cancer: a phase 3 RCT based meta-analysis., PMID:40469183
Atezolizumab plus paclitaxel and bevacizumab as first-line treatment of advanced triple-negative breast cancer: the ATRACTIB phase 2 trial., PMID:40467896
EFFICACY AND PROGNOSIS OF ANTI-VEGF AGENTS COMBINED WITH PANRETINAL PHOTOCOAGULATION IN DIABETIC RETINOPATHY: A CLINICAL OBSERVATIONAL STUDY., PMID:40466685
Dostarlimab and niraparib in primary advanced ovarian cancer., PMID:40461381
Reply to Comment on "Retinal vascularization rate predicts retinopathy of prematurity and remains unaffected by low-dose bevacizumab treatment"., PMID:40460926
Effect of antineoplastic drug therapies on carcinoma and aggressive pituitary tumors: a systematic review and meta-analysis., PMID:40457103
High CD36 expression in the tumor microenvironmental vasculature correlates with unfavorable overall survival in high grade serous ovarian cancer., PMID:40456794
S100B induces angiogenesis via the clathrin/FOXO1/β-catenin signaling pathway and contributes to Bevacizumab resistance in epithelial ovarian cancer., PMID:40456442
Association between intravitreal anti-vascular endothelial growth factor agents and hypertension: a meta-analysis., PMID:40456283
Anti-VEGF Agents Clearance Through the Aqueous Outflow Pathway in a Rat Model., PMID:40455045
Several Common Genetic Variations Associate With Functional or Anatomic Effects of Anti-VEGF Treatment in Conditions With Macular Edema., PMID:40455044