Amino acid substitutions in the fusion protein of respiratory syncytial virus in Fukushima, Japan during 2008-2023 and their effects., PMID:39719011
A potent broad-spectrum neutralizing antibody targeting a conserved region of the prefusion RSV F protein., PMID:39572535
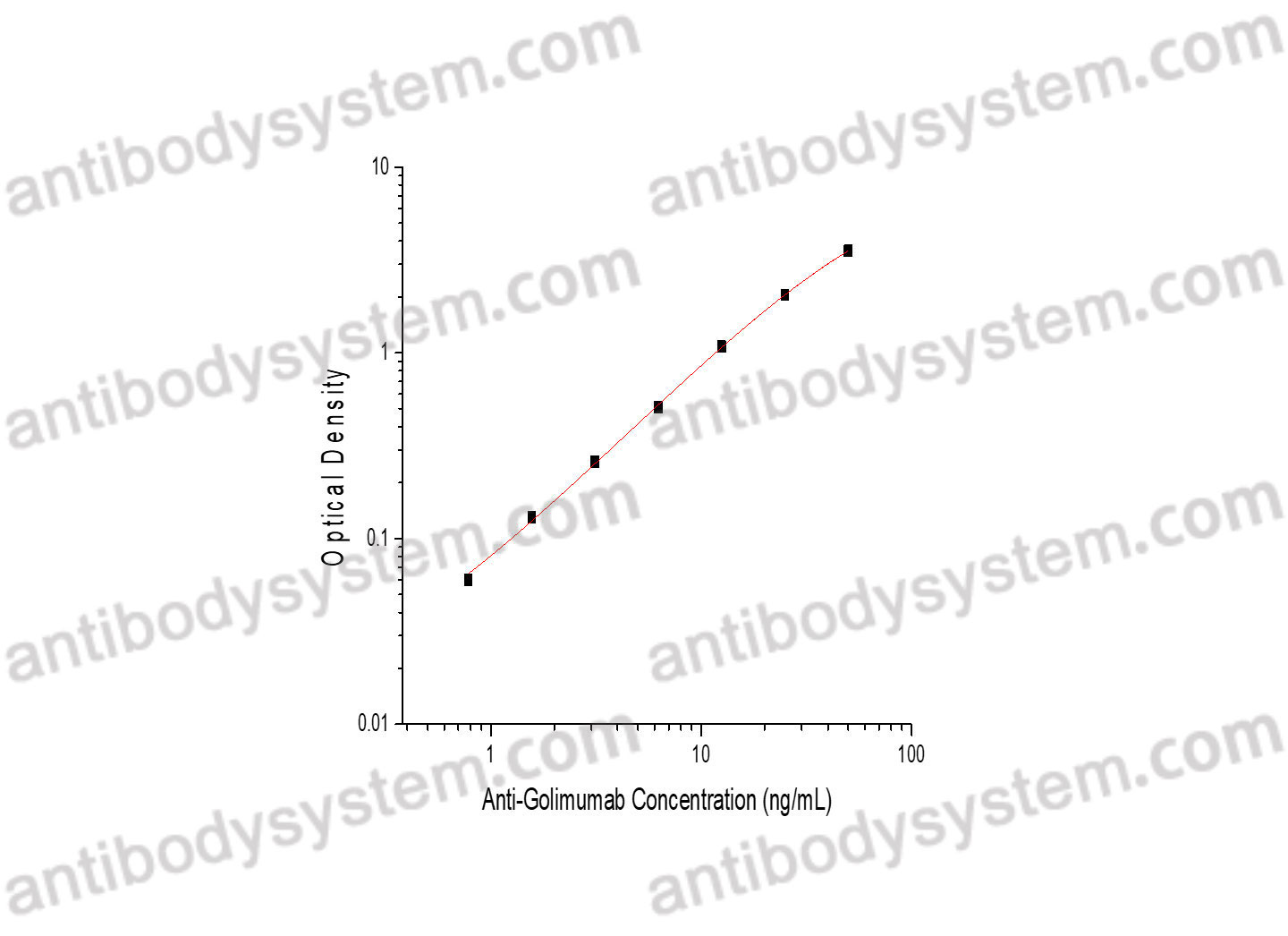
RSV Neutralizing Antibodies Following Nirsevimab and Palivizumab Dosing., PMID:39350745
Molecular and phenotypic characteristics of respiratory syncytial virus isolates recovered from medically vulnerable children: An exploratory analysis of a phase 2/3 randomized, double-blind, palivizumab-controlled trial of nirsevimab (MEDLEY)., PMID:39241352
RSV infection of humanized lung-only mice induces pathological changes resembling severe bronchiolitis and bronchopneumonia., PMID:39175804
[Establishment and preliminary application of neutralizing antibody detection method for human respiratory syncytial virus]., PMID:39034780
Farnesyltransferase inhibitor lonafarnib suppresses respiratory syncytial virus infection by blocking conformational change of fusion glycoprotein., PMID:38853183
Balanced on the Biggest Wave: Nirsevimab for Newborns., PMID:38599778
Monoclonal antibodies targeting sites in respiratory syncytial virus attachment G protein provide protection against RSV-A and RSV-B in mice., PMID:38575575
Neutralizing activity of anti-respiratory syncytial virus monoclonal antibody produced in Nicotiana benthamiana., PMID:38508690
[Prevention of respiratory syncytial virus infection in infants. What has been done and where are we today?]., PMID:38329302
Efficacy of Palivizumab Immunoprophylaxis for Reducing Severe RSV Outcomes in Children with Immunodeficiencies: A Systematic Review., PMID:38279954
Characterization of a Panel of Monoclonal Antibodies Targeting the F-Protein of the Respiratory Syncytial Virus (RSV) for the Typing of Contemporary Circulating Strains., PMID:38276631
Clinical, Genomic, and Immunological Characterization of RSV Surge in Sydney, Australia, 2022., PMID:38225912
Inhaled "Muco-Trapping" Monoclonal Antibody Effectively Treats Established Respiratory Syncytial Virus (RSV) Infections., PMID:38225749
Subtractive Immunization as a Method to Develop Respiratory Syncytial Virus (RSV)-Specific Monoclonal Antibodies., PMID:37873859
The variable conversion of neutralizing anti-SARS-CoV-2 single-chain antibodies to IgG provides insight into RBD epitope accessibility., PMID:37561410
In-vivo and human evidence for potential efficacy of therapeutic polyclonal RSV neutralizing antibodies for palivizumab-resistant RSV infections., PMID:37542818
Immune Prophylaxis Targeting the Respiratory Syncytial Virus (RSV) G Protein., PMID:37243153
[Clinical progress of neutralizing antibodies against SARS-CoV-2]., PMID:35786461
A Randomized Phase 1/2 Study of a Respiratory Syncytial Virus Prefusion F Vaccine., PMID:34932102
Adeno-Associated Virus Vector-Mediated Expression of Antirespiratory Syncytial Virus Antibody Prevents Infection in Mouse Airways., PMID:34415793
RSV neutralization assays - Use in immune response assessment., PMID:34244007
Potent Human Single-Domain Antibodies Specific for a Novel Prefusion Epitope of Respiratory Syncytial Virus F Glycoprotein., PMID:34160257
Epicutaneous immunization using synthetic virus-like particles efficiently boosts protective immunity to respiratory syncytial virus., PMID:34154864
An epitope-specific chemically defined nanoparticle vaccine for respiratory syncytial virus., PMID:34145291
Humoral and Mucosal Antibody Response to RSV Structural Proteins in RSV-Infected Adult Hematopoietic Cell Transplant (HCT) Recipients., PMID:34073490
Monoclonal Antibodies for Prevention of Respiratory Syncytial Virus Infection., PMID:34042909
Dissociation of the respiratory syncytial virus F protein-specific human IgG, IgA and IgM response., PMID:33574352
Effect of digestion on stability of palivizumab IgG1 in the infant gastrointestinal tract., PMID:33214672
A Phase 1 Randomized, Double-Blind, Placebo-Controlled Trial to Assess the Safety, Tolerability, and Pharmacokinetics of a Respiratory Syncytial Virus Neutralizing Monoclonal Antibody MK-1654 in Healthy Adults., PMID:33125189
Autoreactivity of Broadly Neutralizing Influenza Human Antibodies to Human Tissues and Human Proteins., PMID:33049994
Two RSV Platforms for G, F, or G+F Proteins VLPs., PMID:32824936
Respiratory Syncytial Virus and Human Metapneumovirus Infections in Three-Dimensional Human Airway Tissues Expose an Interesting Dichotomy in Viral Replication, Spread, and Inhibition by Neutralizing Antibodies., PMID:32759319
Binding and Neutralizing Capacity of Respiratory Syncytial Virus (RSV)-Specific Recombinant IgG Against RSV in Human Milk, Gastric and Intestinal Fluids from Infants., PMID:32605037
Antibody development for preventing the human respiratory syncytial virus pathology., PMID:32303184
Survival of Recombinant Monoclonal Antibodies (IgG, IgA and sIgA) Versus Naturally-Occurring Antibodies (IgG and sIgA/IgA) in an Ex Vivo Infant Digestion Model., PMID:32120792
Comparative Therapeutic Potential of ALX-0171 and Palivizumab against Respiratory Syncytial Virus Clinical Isolate Infection of Well-Differentiated Primary Pediatric Bronchial Epithelial Cell Cultures., PMID:31767728
Immunogenicity and Safety of 3 Formulations of a Respiratory Syncytial Virus Candidate Vaccine in Nonpregnant Women: A Phase 2, Randomized Trial., PMID:31418022
Respiratory syncytial virus prefusogenic fusion (F) protein nanoparticle vaccine: Structure, antigenic profile, immunogenicity, and protection., PMID:31416644
[Prospects For the Use of Peptides against Respiratory Syncytial Virus]., PMID:31397431
Antigenic Site-Specific Competitive Antibody Responses to the Fusion Protein of Respiratory Syncytial Virus Were Associated With Viral Clearance in Hematopoietic Cell Transplantation Adults., PMID:30984206
Epitope-Specific Serological Assays for RSV: Conformation Matters., PMID:30813394
Boosting subdominant neutralizing antibody responses with a computationally designed epitope-focused immunogen., PMID:30789898
Outcomes related to respiratory syncytial virus with an abbreviated palivizumab regimen in children with congenital heart disease: a descriptive analysis., PMID:30782771
Sequence Analysis of the Fusion Protein Gene of Human Respiratory Syncytial Virus Circulating in China from 2003 to 2014., PMID:30514963
Respiratory syncytial virus fusion nanoparticle vaccine immune responses target multiple neutralizing epitopes that contribute to protection against wild-type and palivizumab-resistant mutant virus challenge., PMID:30389195