Catalog No.
KAJ92751
Description
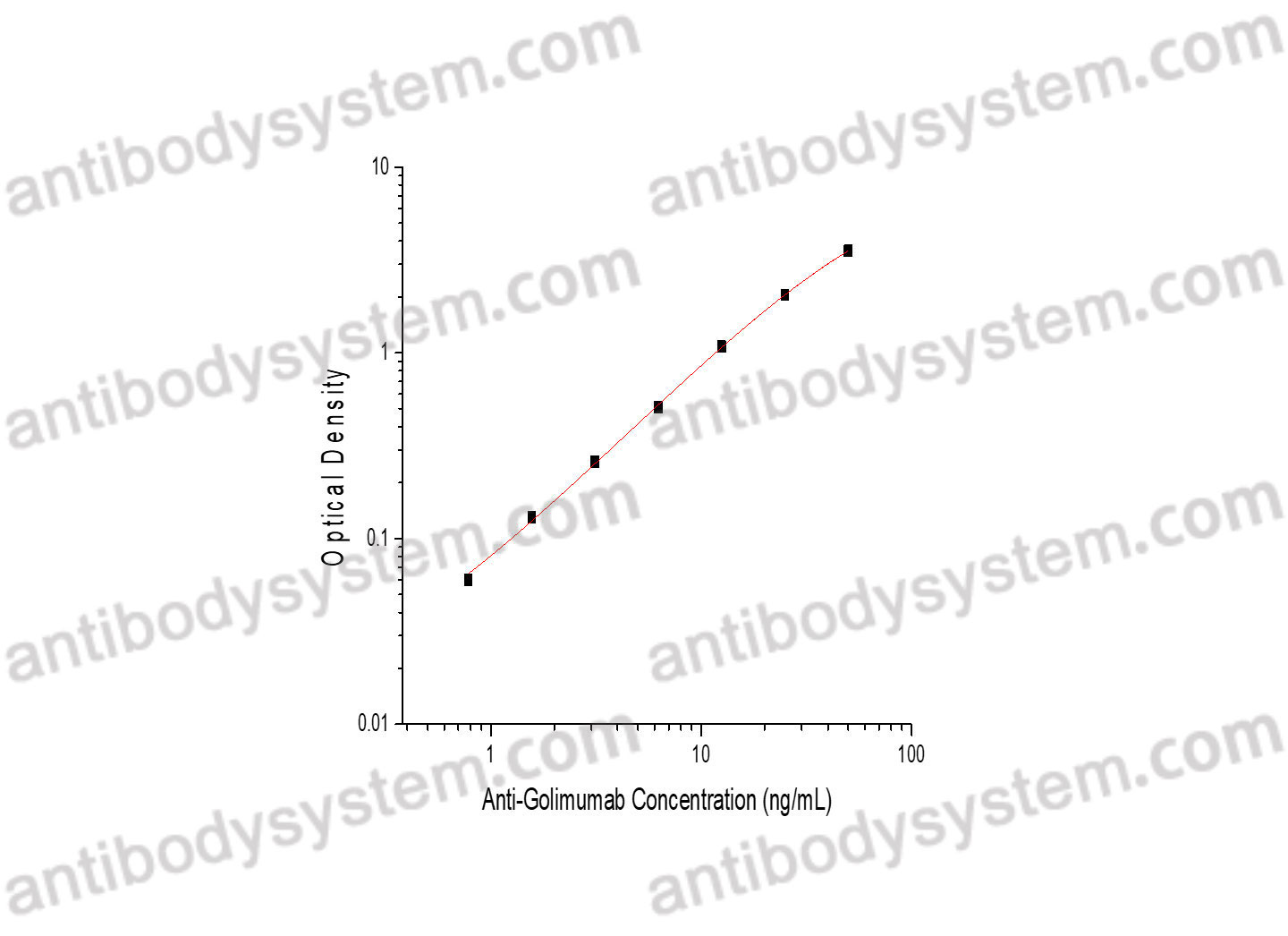
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Omalizumab has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled Human IgE and then pipetted into the wells. Anti-Omalizumab Neutralizing Antibody in the sample competitively binds to Omalizumab with the biotin-labeled Human IgE. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Anti-Omalizumab Neutralizing Antibody bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Omalizumab Neutralizing Antibody concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
156.25 - 10,000 ng/mL
Sensitivity
82.6 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20℃, the rest reagents should be store at 4℃.
Alternative Names
IGE25,olizumab,pSVIE26,rhuMab-E25, CAS: 242138-07-4
Structural and Functional Insights Into IgE Receptor Interactions and Disruptive Inhibition., PMID:40305523
Vaccine-Induced Anti-IgE Antibodies Neutralize Free IgE but Fail to Bind and Activate Mast Cell-Displayed IgE., PMID:40192411
Biological Effects and Clinical Application of the Anti-Immunoglobulin E Antibody., PMID:39551049
A Randomized, Single-Dose, Parallel-Controlled Phase 1 Clinical Comparison of an Omalizumab Biosimilar Candidate with Reference Omalizumab in Healthy Chinese Male Volunteers., PMID:38053476
Combined analytical assays for the characterization of drugs binding to human IgE: Applicability to omalizumab-bearing biosimilar candidates assessment., PMID:37976893
Incidence of Anti-Drug Antibodies to Monoclonal Antibodies in Asthma: A Systematic Review and Meta-Analysis., PMID:36716995
IgE-neutralizing UB-221 mAb, distinct from omalizumab and ligelizumab, exhibits CD23-mediated IgE downregulation and relieves urticaria symptoms., PMID:35912861
Inhibition of immunoglobulin E attenuates pulmonary hypertension., PMID:39196237
Inactivated COVID-19 Vaccine Induces a Low Humoral Immune Response in a Subset of Dermatological Patients Receiving Immunosuppressants., PMID:34957149
Ligelizumab for the treatment of chronic spontaneous urticaria., PMID:32380864
Effectiveness of omalizumab on patient reported outcomes, lung function, and inflammatory markers in severe allergic asthma., PMID:32240649
New biological treatments for asthma and skin allergies., PMID:31444793
Anti-IgE therapy for IgE-mediated allergic diseases: from neutralizing IgE antibodies to eliminating IgE+ B cells., PMID:30026908
Activation of Human Basophils by A549 Lung Epithelial Cells Reveals a Novel IgE-Dependent Response Independent of Allergen., PMID:28652400
A novel IgE-neutralizing antibody for the treatment of severe uncontrolled asthma., PMID:24583620
Immunoglobulin E and Allergy: Antibodies in Immune Inflammation and Treatment., PMID:26184813
Off-label use of omalizumab in non-asthma conditions: new opportunities., PMID:20477322
Decreases in human dendritic cell-dependent T(H)2-like responses after acute in vivo IgE neutralization., PMID:20132969
Experimental approaches towards allergic asthma therapy-murine asthma models., PMID:19891606
[Omalizumab, an anti-IgE monoclonal antibody to treat severe asthma]., PMID:17058881
[Omalizumab, an anti-IgE monoclonal antibody to treat severe asthma]., PMID:16894955
Anti-IgE as a mast cell-stabilizing therapeutic agent., PMID:16750976
Omalizumab : other indications and unanswered questions., PMID:16222081