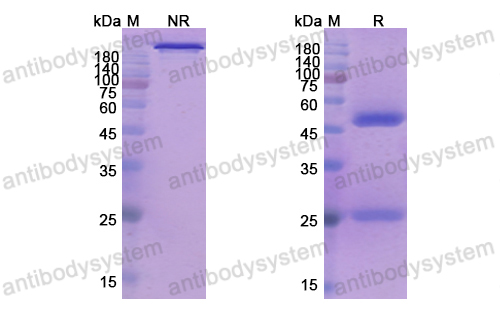
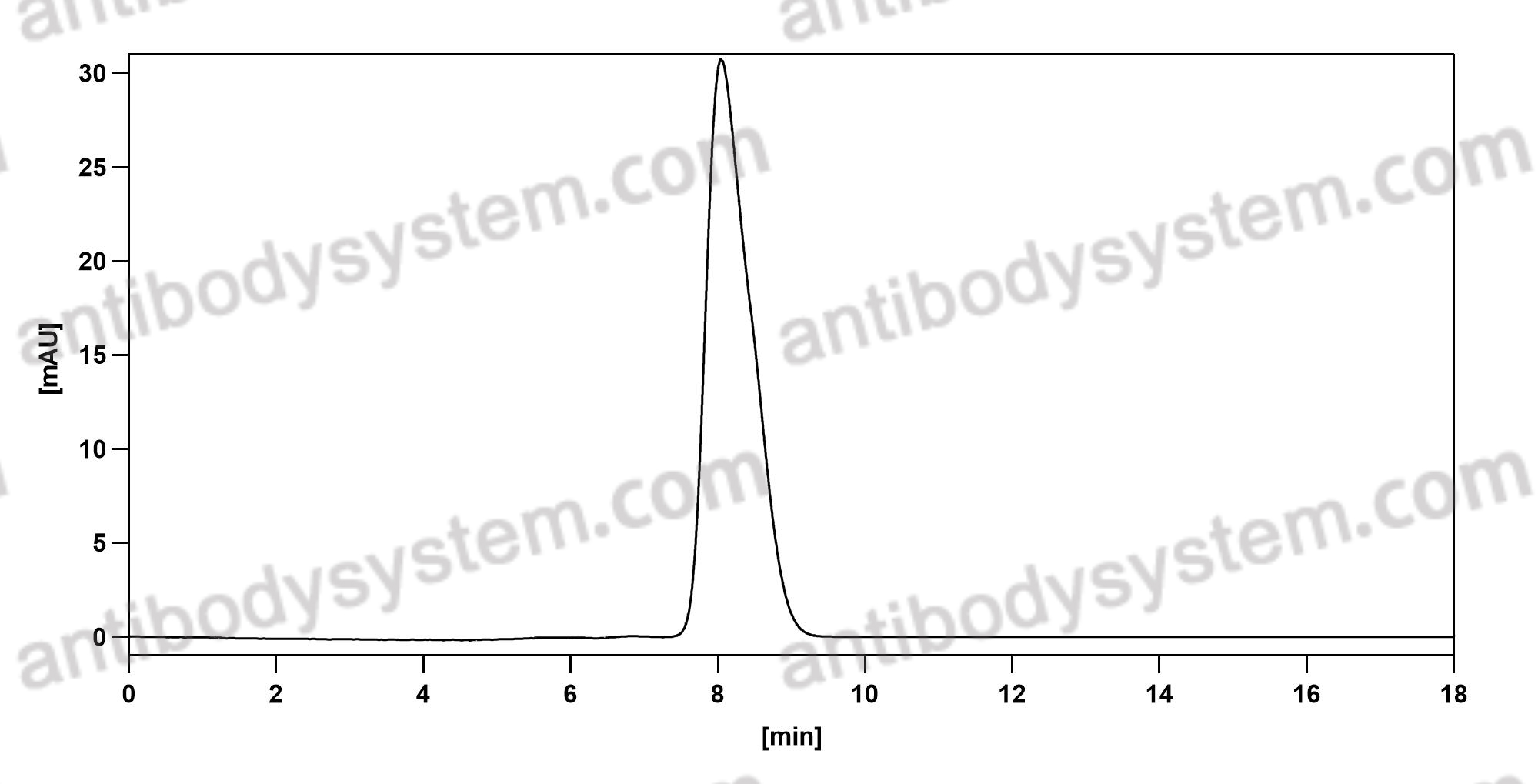
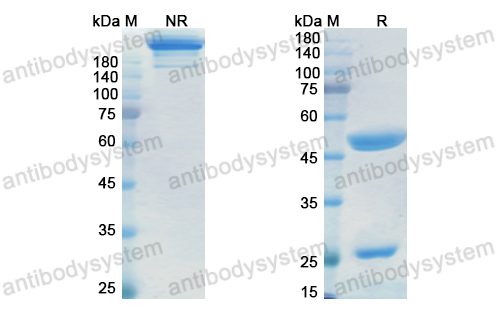
Epratuzumab for the treatment of systemic lupus erythematosus, PMID: 29542345
Epratuzumab for systemic lupus erythematosus, PMID: 23553783
Epratuzumab and Blinatumomab as Therapeutic Antibodies for Treatment of Pediatric Acute Lymphoblastic Leukemia: Current Status and Future Perspectives, PMID: 28088906
The mechanistic impact of CD22 engagement with epratuzumab on B cell function: Implications for the treatment of systemic lupus erythematosus, PMID: 26212727
Epratuzumab: targeting B-cell malignancies through CD22, PMID: 14506198
Epratuzumab in the therapy of oncological and immunological diseases, PMID: 17069520
Profile of epratuzumab and its potential in the treatment of systemic lupus erythematosus, PMID: 25429203
Epratuzumab (humanised anti-CD22 antibody) in autoimmune diseases, PMID: 16918261
Evaluation of epratuzumab as a biologic therapy in systemic lupus erythematosus, PMID: 25496332
Editorial: Epratuzumab: Reveille or Requiem? Teachable Moments for Lupus and Sjögren's Syndrome Clinical Trials, PMID: 29381841
Anti-CD22 epratuzumab for systemic lupus erythematosus: A systematic review and meta-analysis of randomized controlled trials, PMID: 31316634
Efficacy of Epratuzumab, an Anti-CD22 Monoclonal IgG Antibody, in Systemic Lupus Erythematosus Patients With Associated Sjögren's Syndrome: Post Hoc Analyses From the EMBODY Trials, PMID: 29381843
Epratuzumab targeting of CD22 affects adhesion molecule expression and migration of B-cells in systemic lupus erythematosus, PMID: 21050432
Treatment of systemic lupus erythematosus with epratuzumab, PMID: 21219397
Research and therapeutics-traditional and emerging therapies in systemic lupus erythematosus, PMID: 28375452
Emerging B-Cell Therapies in Systemic Lupus Erythematosus, PMID: 33488082
Epratuzumab, a humanized monoclonal antibody targeting CD22: characterization of in vitro properties, PMID: 14506197
Epratuzumab, a CD22-targeting recombinant humanized antibody with a different mode of action from rituximab, PMID: 16814387
Epratuzumab in non-Hodgkin's lymphomas, PMID: 15233905
Preclinical and clinical evaluation of epratuzumab (anti-CD22 IgG) in B-cell malignancies, PMID: 17530024
Epratuzumab inhibits the production of the proinflammatory cytokines IL-6 and TNF-α, but not the regulatory cytokine IL-10, by B cells from healthy donors and SLE patients, PMID: 26183319
Epratuzumab (humanised anti-CD22 antibody) in primary Sjögren's syndrome: an open-label phase I/II study, PMID: 16859536
Epratuzumab for patients with moderate to severe flaring SLE: health-related quality of life outcomes and corticosteroid use in the randomized controlled ALLEVIATE trials and extension study SL0006, PMID: 24273022
Efficacy and Safety of Epratuzumab in Moderately to Severely Active Systemic Lupus Erythematosus: Results From Two Phase III Randomized, Double-Blind, Placebo-Controlled Trials, PMID: 27598855
Trogocytosis of multiple B-cell surface markers by CD22 targeting with epratuzumab, PMID: 23821660
Novel drug targets for personalized precision medicine in relapsed/refractory diffuse large B-cell lymphoma: a comprehensive review, PMID: 26654227
Immunotherapy of non-Hodgkin's lymphoma with hLL2 (epratuzumab, an anti-CD22 monoclonal antibody) and Hu1D10 (apolizumab), PMID: 11842393
Technology evaluation: epratuzumab, Immunomedics/Amgen, PMID: 12772511
Extensive crosslinking of CD22 by epratuzumab triggers BCR signaling and caspase-dependent apoptosis in human lymphoma cells, PMID: 25484043
Safety, pharmacokinetics, and pharmacodynamics of epratuzumab in Japanese patients with moderate-to-severe systemic lupus erythematosus: Results from a phase 1/2 randomized study, PMID: 26382733
Systemic lupus erythematosus: Epratuzumab not effective in phase III trials, PMID: 27652506
Initial clinical trial of epratuzumab (humanized anti-CD22 antibody) for immunotherapy of systemic lupus erythematosus, PMID: 16630358
Re-induction chemoimmunotherapy with epratuzumab in relapsed acute lymphoblastic leukemia (ALL): Phase II results from Children's Oncology Group (COG) study ADVL04P2, PMID: 25732247
Engagement of CD22 on B cells with the monoclonal antibody epratuzumab stimulates the phosphorylation of upstream inhibitory signals of the B cell receptor, PMID: 27125377
Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children's Oncology Group Pilot Study, PMID: 18669463
Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study, PMID: 23313811
Systematic review, and meta-analysis of steroid-sparing effect, of biologic agents in randomized, placebo-controlled phase 3 trials for systemic lupus erythematosus, PMID: 29426575
Efficacy and safety of epratuzumab in patients with moderate/severe flaring systemic lupus erythematosus: results from two randomized, double-blind, placebo-controlled, multicentre studies (ALLEVIATE) and follow-up, PMID: 23542611
Differential effects of epratuzumab on peripheral blood B cells of patients with systemic lupus erythematosus versus normal controls, PMID: 17673490
Long-Term Safety and Efficacy of Epratuzumab in the Treatment of Moderate-to- Severe Systemic Lupus Erythematosus: Results From an Open-Label Extension Study, PMID: 26316325
Epratuzumab-SN-38: a new antibody-drug conjugate for the therapy of hematologic malignancies, PMID: 22039078
(90)Y-labelled anti-CD22 epratuzumab tetraxetan in adults with refractory or relapsed CD22-positive B-cell acute lymphoblastic leukaemia: a phase 1 dose-escalation study, PMID: 26687796
Epratuzumab, a humanized anti-CD22 antibody, in aggressive non-Hodgkin's lymphoma: phase I/II clinical trial results, PMID: 15328168
Anti-CD22 90Y-epratuzumab tetraxetan combined with anti-CD20 veltuzumab: a phase I study in patients with relapsed/refractory, aggressive non-Hodgkin lymphoma, PMID: 25150258
Radioimmunotherapy of non-Hodgkin's lymphoma with 90Y-DOTA humanized anti-CD22 IgG (90Y-Epratuzumab): do tumor targeting and dosimetry predict therapeutic response?, PMID: 14660727
Final results of a phase I radioimmunotherapy trial using (186)Re-epratuzumab for the treatment of patients with non-Hodgkin's lymphoma, PMID: 14506199
Hyper-CVAD + epratuzumab as a salvage regimen for younger patients with relapsed/refractory CD22-positive precursor B-cell acute lymphocytic leukemia, PMID: 28154086
Epratuzumab modulates B-cell signaling without affecting B-cell numbers or B-cell functions in a mouse model with humanized CD22, PMID: 27352780
Dose-fractionated radioimmunotherapy in non-Hodgkin's lymphoma using DOTA-conjugated, 90Y-radiolabeled, humanized anti-CD22 monoclonal antibody, epratuzumab, PMID: 16033839
Characterization of a new humanized anti-CD20 monoclonal antibody, IMMU-106, and Its use in combination with the humanized anti-CD22 antibody, epratuzumab, for the therapy of non-Hodgkin's lymphoma, PMID: 15102696
Asciminib Maintains Antibody-Dependent Cellular Cytotoxicity against Leukemic Blasts., PMID:38610966
Model-based meta-analysis using latent variable modeling to set benchmarks for new treatments of systemic lupus erythematosus., PMID:38050332
Advances in natural products and antibody drugs for SLE: new therapeutic ideas., PMID:37492083
B cell depletion and inhibition in systemic lupus erythematosus., PMID:36342225
Clinical trials in systemic lupus erythematosus: the dilemma-Why have phase III trials failed to confirm the promising results of phase II trials?, PMID:36202589
Immunotherapy in indolent Non-Hodgkin's Lymphoma., PMID:35663281
Direct CNS administration of rituximab and epratuzumab in a pediatric patient with relapsed refractory CNS B-cell acute lymphoblastic leukemia., PMID:35293685
Efficacy of Targeted Immunotherapy as Induction or Salvage Therapy in Acute Lymphoblastic Leukemia: A Systematic Review and Meta-Analysis., PMID:34350787
Distinct patterns of disease activity over time in patients with active SLE revealed using latent class trajectory models., PMID:34321096
Targeting CD22 for the Treatment of B-Cell Malignancies., PMID:34262884
Invasive Fungal Diseases in Children with Hematological Malignancies Treated with Therapies That Target Cell Surface Antigens: Monoclonal Antibodies, Immune Checkpoint Inhibitors and CAR T-Cell Therapies., PMID:33807678
Emerging B-Cell Therapies in Systemic Lupus Erythematosus., PMID:33488082
B cell modulation strategies in the improvement of transplantation outcomes., PMID:32682148
Identification of a moderate affinity CD22 binding peptide and in vitro optimization of peptide-targeted nanoparticles for selective uptake by CD22+ B-cell malignancies., PMID:32436925
The safety and efficacy of biologic agents in treatment of systemic lupus erythematosus: A network meta-analysis., PMID:31777515
The effect of therapies on the quality of life of patients with systemic lupus erythematosus: a meta-analysis of randomized trials., PMID:31358064
Anti-CD22 epratuzumab for systemic lupus erythematosus: A systematic review and meta-analysis of randomized controlled trials., PMID:31316634
The prognostic significance of PFS24 in follicular lymphoma following firstline immunotherapy: A combined analysis of 3 CALGB trials., PMID:30575311
Electrochemically Promoted Tyrosine-Click-Chemistry for Protein Labeling., PMID:30422648
Solution Thermodynamics and Kinetics of Metal Complexation with a Hydroxypyridinone Chelator Designed for Thorium-227 Targeted Alpha Therapy., PMID:30372069
Epratuzumab for the treatment of systemic lupus erythematosus., PMID:29542345
Systematic review, and meta-analysis of steroid-sparing effect, of biologic agents in randomized, placebo-controlled phase 3 trials for systemic lupus erythematosus., PMID:29426575
Efficacy of Epratuzumab, an Anti-CD22 Monoclonal IgG Antibody, in Systemic Lupus Erythematosus Patients With Associated Sjögren's Syndrome: Post Hoc Analyses From the EMBODY Trials., PMID:29381843
Editorial: Epratuzumab: Reveille or Requiem? Teachable Moments for Lupus and Sjögren's Syndrome Clinical Trials., PMID:29381841
Molecular basis of human CD22 function and therapeutic targeting., PMID:28970495
Novel immunotherapies for adult patients with B-lineage acute lymphoblastic leukemia., PMID:28821272
Efficacy and safety of biological DMARDs modulating B cells in primary Sjögren's syndrome: Systematic review and meta-analysis., PMID:28673789
Additional Improvements in Clinical Response From Adjuvant Biologic Response Modifiers in Adults With Moderate to Severe Systemic Lupus Erythematosus Despite Immunosuppressive Agents: A Systematic Review and Meta-analysis., PMID:28673504
Emerging role of immunotherapy in precursor B-cell acute lymphoblastic leukemia., PMID:28666090
Targeting CD22 with the monoclonal antibody epratuzumab modulates human B-cell maturation and cytokine production in response to Toll-like receptor 7 (TLR7) and B-cell receptor (BCR) signaling., PMID:28506291
Biological and targeted therapies of systemic lupus erythematosus: evidence and the state of the art., PMID:28443384
Research and therapeutics-traditional and emerging therapies in systemic lupus erythematosus., PMID:28375452
New Perspectives in Rheumatology: May You Live in Interesting Times: Challenges and Opportunities in Lupus Research., PMID:28371318
Hyper-CVAD + epratuzumab as a salvage regimen for younger patients with relapsed/refractory CD22-positive precursor B-cell acute lymphocytic leukemia., PMID:28154086
Epratuzumab and Blinatumomab as Therapeutic Antibodies for Treatment of Pediatric Acute Lymphoblastic Leukemia: Current Status and Future Perspectives., PMID:28088906
Consolidation anti-CD22 fractionated radioimmunotherapy with 90Y-epratuzumab tetraxetan following R-CHOP in elderly patients with diffuse large B-cell lymphoma: a prospective, single group, phase 2 trial., PMID:27964867
Radioimmunotherapy of lymphoma: an underestimated therapy option., PMID:27964866
Novel Drugs in Follicular Lymphoma., PMID:27872741
Systemic lupus erythematosus: Epratuzumab not effective in phase III trials., PMID:27652506
Biotherapies in systemic lupus erythematosus: New targets., PMID:27663753
Efficacy and Safety of Epratuzumab in Moderately to Severely Active Systemic Lupus Erythematosus: Results From Two Phase III Randomized, Double-Blind, Placebo-Controlled Trials., PMID:27598855
New monoclonal antibodies for the treatment of acute lymphoblastic leukemia., PMID:27521873
Use of Biologics in Sjögren's Syndrome., PMID:27431344
Epratuzumab modulates B-cell signaling without affecting B-cell numbers or B-cell functions in a mouse model with humanized CD22., PMID:27352780
Therapeutic Interventions of Tissue Specific Autoimmune Onset in Systemic Lupus Erythematosus., PMID:27290913
Engagement of CD22 on B cells with the monoclonal antibody epratuzumab stimulates the phosphorylation of upstream inhibitory signals of the B cell receptor., PMID:27125377
Monoclonal antibodies and immune therapies for adult precursor B-acute lymphoblastic leukemia., PMID:26921100
Why, why, why de-lupus (does so badly in clinical trials)., PMID:26786849
(90)Y-labelled anti-CD22 epratuzumab tetraxetan in adults with refractory or relapsed CD22-positive B-cell acute lymphoblastic leukaemia: a phase 1 dose-escalation study., PMID:26687796
Novel drug targets for personalized precision medicine in relapsed/refractory diffuse large B-cell lymphoma: a comprehensive review., PMID:26654227