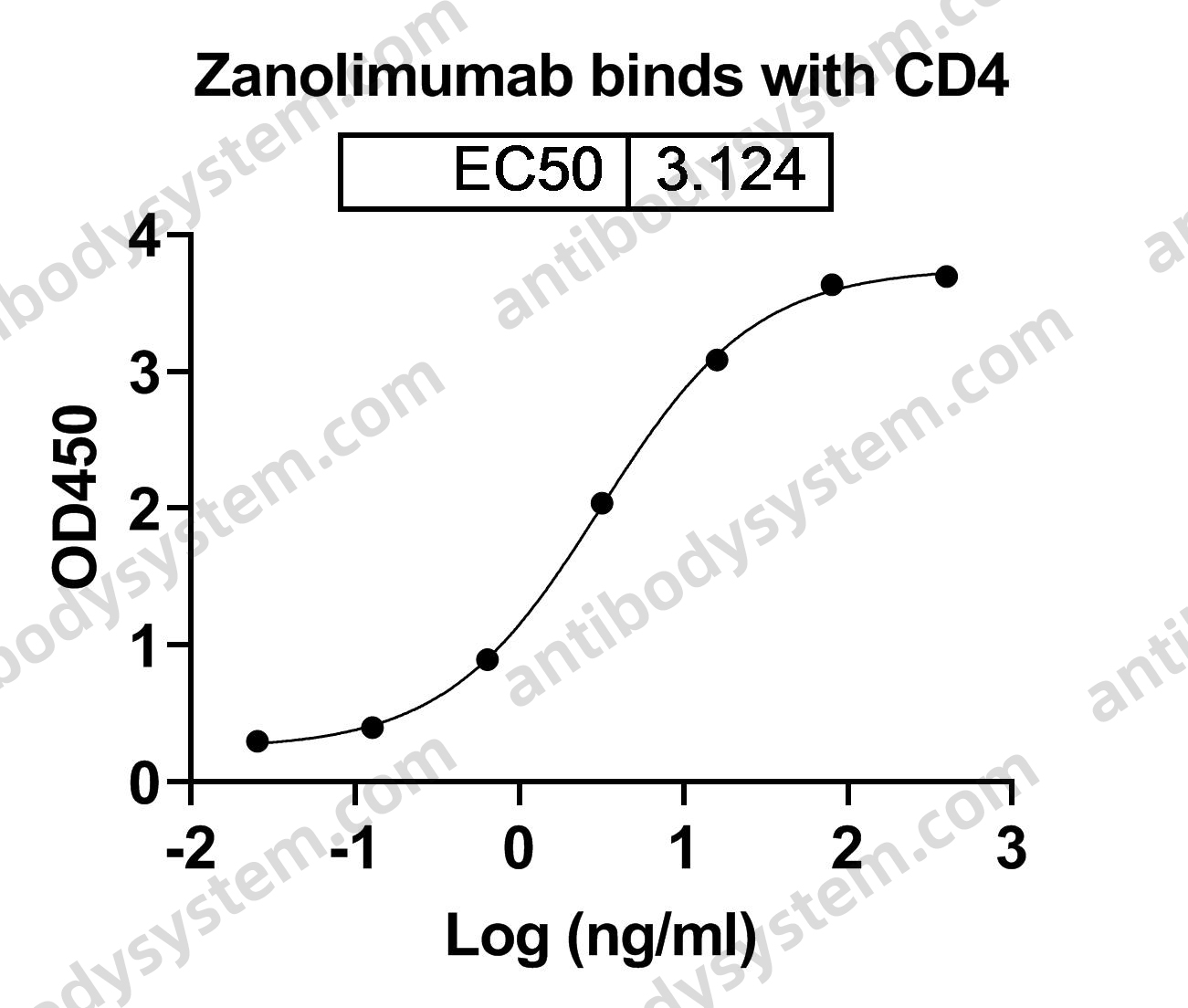
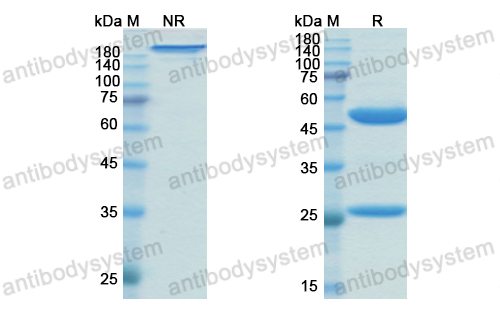
Catalog No.
DHB95902
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
T-cell surface antigen T4/Leu-3, T-cell surface glycoprotein CD4, CD4
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P01730
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
HuMax-CD4, CAS: 652153-01-0
Clone ID
Zanolimumab
Zanolimumab, a human monoclonal antibody targeting CD4 in the treatment of mycosis fungoides and Sézary syndrome, PMID: 18990079
Drug evaluation: zanolimumab, a human monoclonal antibody targeted against CD4, PMID: 17458175
Phase II trial of zanolimumab (HuMax-CD4) in relapsed or refractory non-cutaneous peripheral T cell lymphoma, PMID: 20629661
Alemtuzumab-resistant Sézary syndrome responding to zanolimumab, PMID: 21480857
Clinical efficacy of zanolimumab (HuMax-CD4): two phase 2 studies in refractory cutaneous T-cell lymphoma, PMID: 17311990
In situ depletion of CD4+ T cells in human skin by Zanolimumab, PMID: 17091277
A human CD4 monoclonal antibody for the treatment of T-cell lymphoma combines inhibition of T-cell signaling by a dual mechanism with potent Fc-dependent effector activity, PMID: 17942927
[Primary skin lymphomas: Current therapy], PMID: 30709635
New antibody drug treatments for lymphoma, PMID: 21395497
Gateways to clinical trials, PMID: 16082422
Novel human antibody therapeutics: the age of the Umabs, PMID: 18702090
Newer monoclonal antibodies for hematological malignancies, PMID: 18565392
Novel therapies in peripheral T-cell lymphomas, PMID: 18706268
Cutaneous T-cell lymphomas: Focusing on novel agents in relapsed and refractory disease, PMID: 29102679
Gateways to clinical trials, PMID: 18560631
Antibody-based therapeutics to watch in 2011, PMID: 21051951
Gateways to clinical trials, PMID: 16810345
Gateways to clinical trials, PMID: 16541195
Cutaneous T-cell lymphoma: Biologic targets for therapy, PMID: 20425380
A brief primer on treatments of cutaneous T cell lymphoma, newly approved or late in development, PMID: 17763605
Novel antibodies in the treatment of non-Hodgkin's lymphoma, PMID: 19767657
Molecular biology and targeted therapy of cutaneous T-cell lymphomas, PMID: 19169209
Peripheral T-cell lymphomas: a review of current approaches and hopes for the future, PMID: 23755375
[Biotherapy of auto-immune diseases : past, present and future perspectives], PMID: 19303737
Beyond the guidelines in the treatment of peripheral T-cell lymphoma: new drug development, PMID: 18433608
[Primary skin lymphomas: Current therapy]., PMID:30709635
Cutaneous T-cell lymphomas: Focusing on novel agents in relapsed and refractory disease., PMID:29102679
Peripheral T-cell lymphomas: a review of current approaches and hopes for the future., PMID:23755375
Alemtuzumab-resistant Sézary syndrome responding to zanolimumab., PMID:21480857
New antibody drug treatments for lymphoma., PMID:21395497
Antibody-based therapeutics to watch in 2011., PMID:21051951
Phase II trial of zanolimumab (HuMax-CD4) in relapsed or refractory non-cutaneous peripheral T cell lymphoma., PMID:20629661
Novel antibodies in the treatment of non-Hodgkin's lymphoma., PMID:19767657
[Biotherapy of auto-immune diseases : past, present and future perspectives]., PMID:19303737
Molecular biology and targeted therapy of cutaneous T-cell lymphomas., PMID:19169209
Zanolimumab, a human monoclonal antibody targeting CD4 in the treatment of mycosis fungoides and Sézary syndrome., PMID:18990079
Novel therapies in peripheral T-cell lymphomas., PMID:18706268
Novel human antibody therapeutics: the age of the Umabs., PMID:18702090
Newer monoclonal antibodies for hematological malignancies., PMID:18565392
Gateways to clinical trials., PMID:18560631
Beyond the guidelines in the treatment of peripheral T-cell lymphoma: new drug development., PMID:18433608
A human CD4 monoclonal antibody for the treatment of T-cell lymphoma combines inhibition of T-cell signaling by a dual mechanism with potent Fc-dependent effector activity., PMID:17942927
Cutaneous T-cell lymphoma: Biologic targets for therapy., PMID:20425380
A brief primer on treatments of cutaneous T cell lymphoma, newly approved or late in development., PMID:17763605
Drug evaluation: zanolimumab, a human monoclonal antibody targeted against CD4., PMID:17458175
Clinical efficacy of zanolimumab (HuMax-CD4): two phase 2 studies in refractory cutaneous T-cell lymphoma., PMID:17311990
In situ depletion of CD4+ T cells in human skin by Zanolimumab., PMID:17091277
Gateways to clinical trials., PMID:16810345
Gateways to clinical trials., PMID:16541195
Gateways to clinical trials., PMID:16082422