Characterization of the vaccinia virus A35R protein and its role in virulence. PMID: 16352555
Enhancing the Immunogenicity of Vaccinia Virus. PMID: 35891430
Vaccinia virus A35R inhibits MHC class II antigen presentation. PMID: 19954808
Monkeypox infection elicits strong antibody and B cell response against A35R and H3L antigens. PMID: 36687315
Mpox vaccine and infection-driven human immune signatures. PMID: 36945651
Experimental Evolution Identifies Vaccinia Virus Mutations in A24R and A35R That Antagonize the Protein Kinase R Pathway and Accompany Collapse of an Extragenic Gene Amplification. PMID: 26202237
Rational development of multicomponent mRNA vaccine candidates against mpox. PMID: 36947428
Enhancing the Protective Immune Response to Administration of a LIVP-GFP Live Attenuated Vaccinia Virus to Mice. PMID: 33801026
Development of multi-epitope vaccines against the monkeypox virus based on envelope proteins using immunoinformatics approaches. PMID: 36993967
Three neutralizing mAbs induced by MPXV A29L protein recognizing different epitopes act synergistically against orthopoxvirus. PMID: 37288876
Heterogeneity in the A33 protein impacts the cross-protective efficacy of a candidate smallpox DNA vaccine. PMID: 18482742
Highly immunogenic variant of attenuated vaccinia virus. PMID: 27025484
Monkeypox virus quadrivalent mRNA vaccine induces immune response and protects against vaccinia virus. PMID: 37117161
Design peptide and multi-epitope protein vaccine candidates against monkeypox virus using reverse vaccinology approach: an in-silico study. PMID: 37154825
Vaccinia virus egress mediated by virus protein A36 is reliant on the F12 protein. PMID: 28631604
Deletion of the A35 gene from Modified Vaccinia Virus Ankara increases immunogenicity and isotype switching. PMID: 21352940
Immunogenicity of MVA-BN vaccine deployed as mpox prophylaxis: a prospective, single-centre, cohort study and analysis of transcriptomic predictors of response., PMID:40286799
The two-dose MVA-BN mpox vaccine induces a nondurable and low avidity MPXV-specific antibody response., PMID:40162783
Rational mpox vaccine design: immunogenicity and protective effect of individual and multicomponent proteins in mice., PMID:40105863
Development and validation of a quantitative Orthopoxvirus immunoassay to evaluate and differentiate serological responses to Mpox infection and vaccination., PMID:39987746
Short-term evolution of Mpox-specific IgG and neutralizing antibodies among individuals undergoing MVA-BN vaccination., PMID:39894441
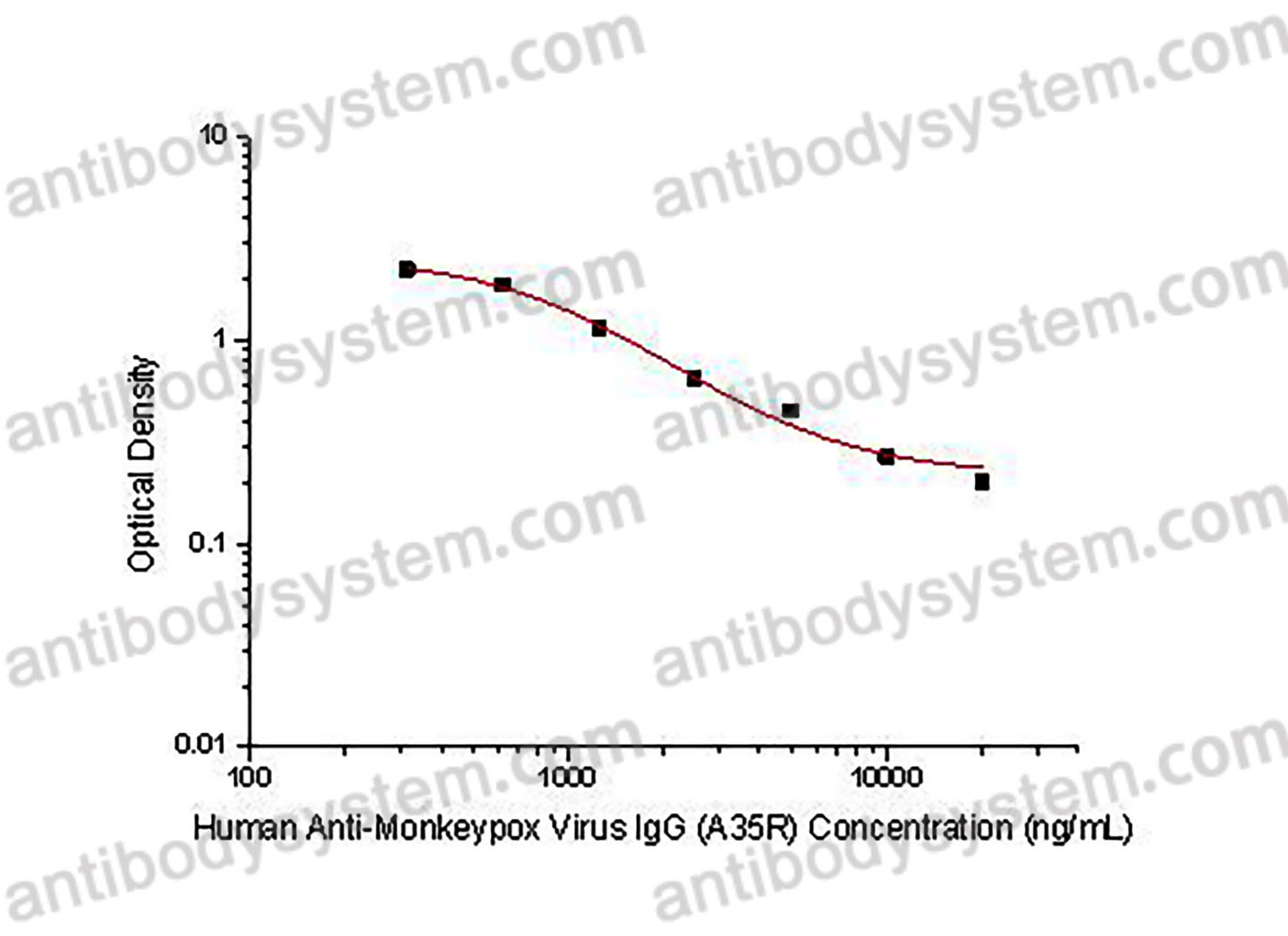
Enhanced Immunogenicity and Affinity with A35R-Fc-Based Chimeric Protein Compared to MPXV A35R Protein., PMID:39861905
Assessment of MpoxPlex, a high-throughput and multiplexed immunoassay: a diagnostic accuracy study., PMID:39832516
Short-Lived Neutralizing Antibody Responses to Monkeypox Virus in Smallpox Vaccine-Naive Persons after JYNNEOS Vaccination., PMID:39793541
An mpox quadrivalent mRNA vaccine elicits sustained and protective immunity in mice against lethal vaccinia virus challenge., PMID:39745170
Long-Lasting Protection and Dose Optimization of MPXV Polyvalent Mpox mRNA Vaccines Against Lethal Vaccinia Virus Challenge in Mice., PMID:39726255
Elderly Individuals Exhibit Elevated Levels of Anti-Monkeypox Virus Antibodies Compared to Adults, Youth, and Children., PMID:39569454
Unique Kinetics of the Human Milk Antibody Response to JYNNEOS Vaccine for Prevention of Monkey Pox: A Case Study., PMID:39360771
Rational design and computational evaluation of a multi-epitope vaccine for monkeypox virus: Insights into binding stability and immunological memory., PMID:39247273
Investigating seroprevalence of IgG antibodies against Monkeypox Virus (MPXV) in a cohort of people living with HIV (PLWH) in Rome, during the 2022 outbreak: Moving beyond traditional at-risk populations., PMID:39241937
An Attenuated and Highly Immunogenic Variant of the Vaccinia Virus., PMID:39188266
Profiling of viral load, antibody and inflammatory response of people with monkeypox during hospitalization: a prospective longitudinal cohort study in China., PMID:39043012
Clinical characteristics, viral dynamics, and antibody response of monkeypox virus infections among men with and without HIV infection in Guangzhou, China., PMID:38979508
Trimerized S expressed by modified vaccinia virus Ankara (MVA) confers superior protection against lethal SARS-CoV-2 challenge in mice., PMID:38874361
Immune responses associated with mpox viral clearance in men with and without HIV in Spain: a multisite, observational, prospective cohort study., PMID:38857615
Third-generation smallpox vaccines induce low-level cross-protecting neutralizing antibodies against Monkeypox virus in laboratory workers., PMID:38826712
Longitudinal viral shedding and antibody response characteristics of men with acute infection of monkeypox virus: a prospective cohort study., PMID:38802350
Antibodies Induced by Smallpox Vaccination after at Least 45 Years Cross-React with and In Vitro Neutralize Mpox Virus: A Role for Polyclonal B Cell Activation?, PMID:38675961
Scenarios of future mpox outbreaks among men who have sex with men: a modelling study based on cross-sectional seroprevalence data from the Netherlands, 2022., PMID:38666400
Mpox outbreak in Rio de Janeiro, Brazil: A translational approach., PMID:38654686
Breakthrough Mpox Outbreak Investigation, the Delicate Balance Between Host Immune Response and Viral Immune Escape., PMID:38647249
A multivalent mRNA monkeypox virus vaccine (BNT166) protects mice and macaques from orthopoxvirus disease., PMID:38366591
Characterizing the acute antibody response of monkeypox and MVA-BN vaccine following an Australian outbreak., PMID:38240403
Review of virological methods for laboratory diagnosis and characterization of monkeypox virus (MPXV): lessons learned from the 2022 Mpox outbreak., PMID:38179904
Serological methods for the detection of antibodies against monkeypox virus applicable for laboratories with different biosafety levels., PMID:38054557
Serosurvey of Immunity to Monkeypox (Mpox) Virus Antigens in People Living with HIV in South Florida., PMID:38003819
Smallpox vaccination in a mouse model., PMID:37965374
Prevalence of Undiagnosed Monkeypox Virus Infections during Global Mpox Outbreak, United States, June-September 2022., PMID:37832516
Mpox as AIDS-defining event with a severe and protracted course: clinical, immunological, and virological implications., PMID:37778364
Duration of humoral immunity from smallpox vaccination and its cross-reaction with Mpox virus., PMID:37709783
A Monoclonal Antibody Produced in Glycoengineered Plants Potently Neutralizes Monkeypox Virus., PMID:37514995
Mpox virus mRNA-lipid nanoparticle vaccine candidates evoke antibody responses and drive protection against the Vaccinia virus challenge in mice., PMID:37429529
Cross-reactive antibodies against monkeypox virus exist in the population immunized with vaccinia Tian Tan strain in China., PMID:37392823
Early evaluation of the safety, reactogenicity, and immune response after a single dose of modified vaccinia Ankara-Bavaria Nordic vaccine against mpox in children: a national outbreak response., PMID:37336224
Overview of Diagnostic Methods, Disease Prevalence and Transmission of Mpox (Formerly Monkeypox) in Humans and Animal Reservoirs., PMID:37317160
Multi-valent mRNA vaccines against monkeypox enveloped or mature viron surface antigens demonstrate robust immune response and neutralizing activity., PMID:37300753
Profiling the acute phase antibody response against mpox virus in patients infected during the 2022 outbreak., PMID:37287343
Computational Vaccine Design for Poxviridae Family Viruses., PMID:37258933
Serological responses to smallpox A33 antigen and monkeypox A35 antigen in healthy people and people living with HIV-1 from Guangzhou, China., PMID:37212313
Monkeypox virus quadrivalent mRNA vaccine induces immune response and protects against vaccinia virus., PMID:37117161
Proteomics-based vaccine targets annotation and design of subunit and mRNA-based vaccines for Monkeypox virus (MPXV) against the recent outbreak., PMID:37116237
Monkeypox (mpox) in immunosuppressed patients., PMID:37089133
Clinical characterization and placental pathology of mpox infection in hospitalized patients in the Democratic Republic of the Congo., PMID:37079637
Design and Optimization of a Monkeypox virus Specific Serological Assay., PMID:36986317
Kinetics of viral DNA in body fluids and antibody response in patients with acute Monkeypox virus infection., PMID:36748085
Comparison of the Effectiveness of Transepidemal and Intradermal Immunization of Mice with the Vacinia Virus., PMID:36694907