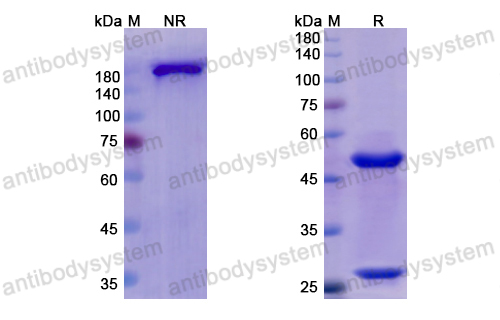
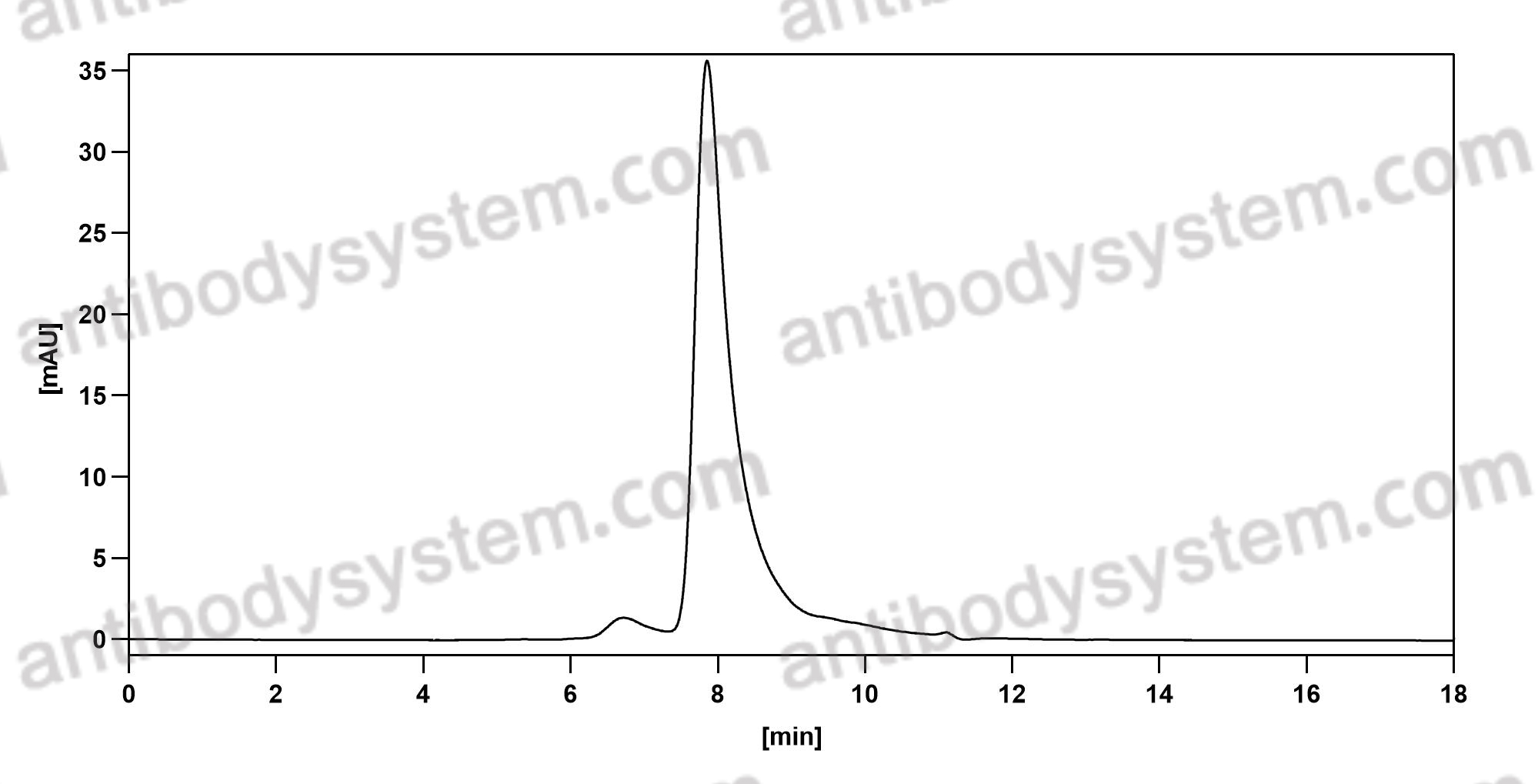
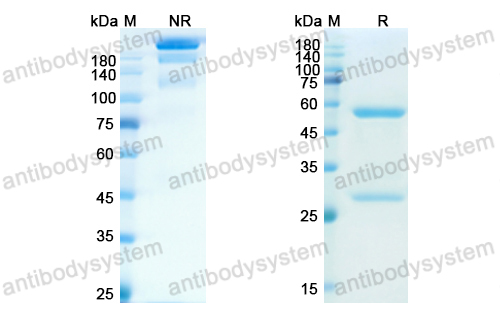
Otelixizumab: a novel agent for the prevention of type 1 diabetes mellitus, PMID: 21913874
Efficacy and safety of otelixizumab use in new-onset type 1 diabetes mellitus, PMID: 27145230
Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside, PMID: 27161438
A randomised, single-blind, placebo-controlled, dose-finding safety and tolerability study of the anti-CD3 monoclonal antibody otelixizumab in new-onset type 1 diabetes, PMID: 33145642
Target engagement and cellular fate of otelixizumab: a repeat dose escalation study of an anti-CD3ε mAb in new-onset type 1 diabetes mellitus patients, PMID: 30566758
Targeting T cells in inflammatory bowel disease, PMID: 32585338
Otelixizumab in the treatment of type 1 diabetes mellitus, PMID: 22053883
Molecule of the month. Otelixizumab, PMID: 20520855
Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: results of the randomized phase III study in recent-onset human type 1 diabetes, PMID: 25011949
Subcutaneous Administration of Otelixizumab is Limited by Injection Site Reactions: Results of an Exploratory Study in Type 1 Diabetes Mellitus Patients, PMID: 27023009
Pharmacokinetics and antibody responses to the CD3 antibody otelixizumab used in the treatment of type 1 diabetes, PMID: 20147616
Temporal pharmacokinetic/pharmacodynamic interaction between human CD3ε antigen-targeted monoclonal antibody otelixizumab and CD3ε binding and expression in human peripheral blood mononuclear cell static culture, PMID: 26341624
Pharmacokinetics and pharmacodynamics of a chimeric/humanized anti-CD3 monoclonal antibody, otelixizumab (TRX4), in subjects with psoriasis and with type 1 diabetes mellitus, PMID: 19934031
Urinary C-peptide analysis in an intervention study: experience from the DEFEND-2 otelixizumab trial, PMID: 26871270
Preexisting insulin autoantibodies predict efficacy of otelixizumab in preserving residual β-cell function in recent-onset type 1 diabetes, PMID: 25583753
Efficacy and safety of low-dose otelixizumab anti-CD3 monoclonal antibody in preserving C-peptide secretion in adolescent type 1 diabetes: DEFEND-2, a randomized, placebo-controlled, double-blind, multi-centre study, PMID: 24236828
Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial, PMID: 21719095
A CD3-specific antibody reduces cytokine production and alters phosphoprotein profiles in intestinal tissues from patients with inflammatory bowel disease, PMID: 24704524
Prospects for therapeutic tolerance in humans, PMID: 24378931
Anti-CD3 clinical trials in type 1 diabetes mellitus, PMID: 23726024
Combination therapies in the context of anti-CD3 antibodies for the treatment of autoimmune diseases, PMID: 23254986
Immunotherapies in diabetes mellitus type 1, PMID: 22703858
Emerging treatments for the prevention of type 1 diabetes, PMID: 20384544
Anti-CD3 mAbs for treatment of type 1 diabetes, PMID: 19319985
Oral histone deacetylase inhibitor synergises with T cell targeted immunotherapy to preserve beta cell metabolic function and induce stable remission of new-onset autoimmune diabetes in NOD mice, PMID: 29030662
Anti-CD3 Antibody for the Prevention of Type 1 Diabetes: A Story of Perseverance, PMID: 31523950
CD3 monoclonal antibodies: a first step towards operational immune tolerance in the clinic, PMID: 23804274
Clinical update on the use of immuno modulators (antiCD3, GAD, Diapep277, anti-IL1) in type 1 diabetes, PMID: 21864261
Immunomodulators: Cell savers, PMID: 22616095
Type 1 diabetes pathogenesis - Prevention???, PMID: 25941654
Antibody-based therapeutics to watch in 2011, PMID: 21051951
Biologic therapies in type 1 diabetes: how far are they from us?, PMID: 24229664
Human CD3 transgenic mice: preclinical testing of antibodies promoting immune tolerance, PMID: 21289272
Preservation of recall immunity in anti-CD3-treated recent onset type 1 diabetes patients, PMID: 22069286
Studies in human intestinal tissues: is it time to reemphasize research in human immunology?, PMID: 24877864
Elevated T cell levels in peripheral blood predict poor clinical response following rituximab treatment in new-onset type 1 diabetes, PMID: 29925930
Anti-CD3 antibodies for type 1 diabetes: beyond expectations, PMID: 21719098
Prevention and reversal of type 1 diabetes--past challenges and future opportunities, PMID: 25998292
Development of a quantitative assay to measure EBV viral load in patients with autoimmune type 1 diabetes and healthy subjects, PMID: 19931313
Development of a stable low-dose aglycosylated antibody formulation to minimize protein loss during intravenous administration, PMID: 26073995
Methods to measure the binding of therapeutic monoclonal antibodies to the human Fc receptor FcγRIII (CD16) using real time kinetic analysis and flow cytometry, PMID: 22366323
[Antibodies instead of insulin?], PMID: 20196056
Immunotherapy as a treatment for type 1 diabetes mellitus in children and young adults: A comprehensive systematic review and meta-analysis., PMID:40215252
Identifying Promising Immunomodulators for Type 1 Diabetes (T1D) and Islet Transplantation., PMID:39735417
Effect of Immunotherapy on C-peptide Levels in Patients With Type I Diabetes Mellitus: A Systematic Review of Randomized Controlled Trials., PMID:38800168
Baseline plasma proinsulin response to glucose for predicting therapeutic response to otelixizumab in recent-onset type 1 diabetes., PMID:37884063
Anti-CD3 monoclonal antibodies in treatment of type 1 diabetes: a systematic review and meta-analysis., PMID:37658243
Efficacy of anti-CD3 monoclonal antibodies in delaying the progression of recent-onset type 1 diabetes mellitus: A systematic review, meta-analyses and meta-regression., PMID:37580969
Anti-CD3 monoclonal antibodies for the prevention and treatment of type 1 diabetes: A literature review., PMID:36056809
Investigational therapies targeting CD3 for prevention and treatment of type 1 diabetes., PMID:34936848
A randomised, single-blind, placebo-controlled, dose-finding safety and tolerability study of the anti-CD3 monoclonal antibody otelixizumab in new-onset type 1 diabetes., PMID:33145642
Targeting T cells in inflammatory bowel disease., PMID:32585338
Anti-CD3 Antibody for the Prevention of Type 1 Diabetes: A Story of Perseverance., PMID:31523950
Target engagement and cellular fate of otelixizumab: a repeat dose escalation study of an anti-CD3ε mAb in new-onset type 1 diabetes mellitus patients., PMID:30566758
Elevated T cell levels in peripheral blood predict poor clinical response following rituximab treatment in new-onset type 1 diabetes., PMID:29925930
Oral histone deacetylase inhibitor synergises with T cell targeted immunotherapy to preserve beta cell metabolic function and induce stable remission of new-onset autoimmune diabetes in NOD mice., PMID:29030662
Urinary C-peptide analysis in an intervention study: experience from the DEFEND-2 otelixizumab trial., PMID:26871270
Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside., PMID:27161438
Efficacy and safety of otelixizumab use in new-onset type 1 diabetes mellitus., PMID:27145230
Subcutaneous Administration of Otelixizumab is Limited by Injection Site Reactions: Results of an Exploratory Study in Type 1 Diabetes Mellitus Patients., PMID:27023009
Temporal pharmacokinetic/pharmacodynamic interaction between human CD3ε antigen-targeted monoclonal antibody otelixizumab and CD3ε binding and expression in human peripheral blood mononuclear cell static culture., PMID:26341624
Development of a stable low-dose aglycosylated antibody formulation to minimize protein loss during intravenous administration., PMID:26073995
Prevention and reversal of type 1 diabetes--past challenges and future opportunities., PMID:25998292
Type 1 diabetes pathogenesis - Prevention???, PMID:25941654
Preexisting insulin autoantibodies predict efficacy of otelixizumab in preserving residual β-cell function in recent-onset type 1 diabetes., PMID:25583753
Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: results of the randomized phase III study in recent-onset human type 1 diabetes., PMID:25011949
Studies in human intestinal tissues: is it time to reemphasize research in human immunology?, PMID:24877864
A CD3-specific antibody reduces cytokine production and alters phosphoprotein profiles in intestinal tissues from patients with inflammatory bowel disease., PMID:24704524
Prospects for therapeutic tolerance in humans., PMID:24378931
Efficacy and safety of low-dose otelixizumab anti-CD3 monoclonal antibody in preserving C-peptide secretion in adolescent type 1 diabetes: DEFEND-2, a randomized, placebo-controlled, double-blind, multi-centre study., PMID:24236828
Biologic therapies in type 1 diabetes: how far are they from us?, PMID:24229664
Anti-CD3 clinical trials in type 1 diabetes mellitus., PMID:23726024
Combination therapies in the context of anti-CD3 antibodies for the treatment of autoimmune diseases., PMID:23254986
Immunotherapies in diabetes mellitus type 1., PMID:22703858
Immunomodulators: Cell savers., PMID:22616095
Methods to measure the binding of therapeutic monoclonal antibodies to the human Fc receptor FcγRIII (CD16) using real time kinetic analysis and flow cytometry., PMID:22366323
CD3 monoclonal antibodies: a first step towards operational immune tolerance in the clinic., PMID:23804274
Preservation of recall immunity in anti-CD3-treated recent onset type 1 diabetes patients., PMID:22069286
Otelixizumab in the treatment of type 1 diabetes mellitus., PMID:22053883
Otelixizumab: a novel agent for the prevention of type 1 diabetes mellitus., PMID:21913874
Clinical update on the use of immuno modulators (antiCD3, GAD, Diapep277, anti-IL1) in type 1 diabetes., PMID:21864261
Anti-CD3 antibodies for type 1 diabetes: beyond expectations., PMID:21719098
Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial., PMID:21719095
Human CD3 transgenic mice: preclinical testing of antibodies promoting immune tolerance., PMID:21289272
Antibody-based therapeutics to watch in 2011., PMID:21051951
Molecule of the month. Otelixizumab., PMID:20520855
Emerging treatments for the prevention of type 1 diabetes., PMID:20384544
[Antibodies instead of insulin?]., PMID:20196056
Pharmacokinetics and antibody responses to the CD3 antibody otelixizumab used in the treatment of type 1 diabetes., PMID:20147616
Pharmacokinetics and pharmacodynamics of a chimeric/humanized anti-CD3 monoclonal antibody, otelixizumab (TRX4), in subjects with psoriasis and with type 1 diabetes mellitus., PMID:19934031
Development of a quantitative assay to measure EBV viral load in patients with autoimmune type 1 diabetes and healthy subjects., PMID:19931313
Anti-CD3 mAbs for treatment of type 1 diabetes., PMID:19319985