Catalog No.
PAJ92701
Host species
Rabbit
Isotype
IgG
Clonality
Polyclonal
Immunogen
Omalizumab
Tested applications
ELISA: 1:4000-1:8000
Target
Rabbit polyclonal to Xolair.
Specificity
The product is specific for Omalizumab. This antibody serves as an excellent positive control for Omalizumab immunogenicity (ADA) assays.
Concentration
0.41 mg/ml
Purification
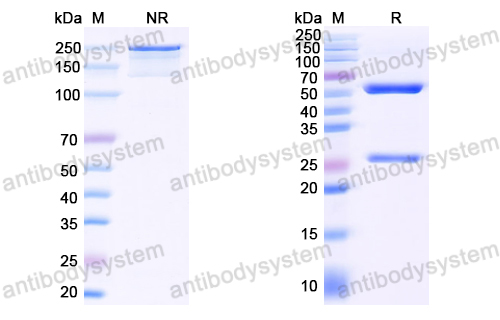
Purified by antigen affinity column.
Accession
CAS: 242138-07-4
Applications
ELISA
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4, 50% Glycerol, 0.05% Proclin 300.
Stability and Storage
The product can be stored for 2 - 3 weeks at 2 to 8°C or for up to 12 months at -20°C. Avoid repeated freezing and thawing cycles.
Background
Omalizumab is a recombinant, humanized, monoclonal antibody against human immunoglobulin E (IgE). It is derived from a murine monoclonal antibody, which was humanized to produce Omalizumab in its present form. It contains 5% non-human (murine) amino acid residues, which make up the complementarity determining regions (CDRs) of the protein.Antibodysystem Anti-Omalizumab Antibody, pAb, Rabbit is produced from sera of a rabbit immunized with Omalizumab.
Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Immunogenicity Profiles of a Single-Dose JYB1904 in Healthy Chinese Participants: A Phase Ia, Randomized, Double-Blind, Placebo-Controlled Study., PMID:40504164
A nationwide experience of biological treatments in children with eosinophilic esophagitis., PMID:40483369
How to manage hypersensitivity reactions to enzyme replacement therapy in lysosomal storage diseases?, PMID:40481554
Evaluating remibrutinib in the treatment of chronic spontaneous urticaria., PMID:40455080
Hereditary alpha-tryptasemia - a potential cause of severe anaphylactic reactions and a modifier of mast cell diseases., PMID:40450750
Efficacy and safety of Omalizumab in the treatment of CSU: a meta-analysis of a randomized controlled trial., PMID:40439896
Omalizumab Efficacy in Chronic Rhinosinusitis Patients with Recurrent Nasal Polyps: An open-label, single-center, randomized, controlled study., PMID:40437843
Omalizumab in Food Allergy in Children: Current Evidence and Future Perspectives., PMID:40430110
Anti-KIT Barzolvolimab for Chronic Spontaneous Urticaria., PMID:40415544
Biologic Therapies for Severe Asthma: Current Insights and Future Directions., PMID:40364184
Omalizumab: a broader role in dermatology? Evidence and the road ahead., PMID:40338196
Pityriasis rosea-like eruption induced by omalizumab: a case report of a rare side effect., PMID:40330944
Scientific developments in understanding food allergy prevention, diagnosis, and treatment., PMID:40330465
Spectrum and Impact of Reported Side Effects of Omalizumab in Patients With Chronic Urticaria: A Long-Term Multicentre Real-World Study., PMID:40325793
Glycobiology of IgE., PMID:40322856
Structural and Functional Insights Into IgE Receptor Interactions and Disruptive Inhibition., PMID:40305523
Efficacy of tiotropium bromide on spirometric measurements and control of asthma in real life: data from a 1-year clinical follow-up., PMID:40304436
Secukinumab as a Novel Treatment for Chronic Netherton Syndrome in a Young Adult., PMID:40299794
Research progress of anti-IGE treatment for allergic rhinitis., PMID:40286545
Anti-thyroid peroxidase antibody (anti-TPO antibodies) is higher in children with chronic urticaria who require omalizumab., PMID:40274229
Real-world clinical utility (effectiveness) of omalizumab as add-on therapy in patient with difficult-to-treat severe allergic asthma., PMID:40270877
Sinonasal Intervention Reduces the Need for Pressure Equalization Tube Placement in Atopic Adults., PMID:40265713
Diffusion dimensionality modeling of subcutaneous/intramuscular absorption of antibodies and long-acting injectables., PMID:40263185
[Pharmacoeconomic evaluation of preseasonal treatment of omalizumab in seasonal allergic rhinitis]., PMID:40262981
Efficacy of Biologics in Reducing Exacerbations Requiring Hospitalization or an Emergency Department Visit in Patients with Moderate or Severe, Uncontrolled Asthma., PMID:40261563
Quality of life, sleep, and psychological well-being in chronic spontaneous urticaria patients receiving omalizumab: a case-control study., PMID:40244437
Free-IgE as a Predictor of Responsiveness to Omalizumab in Oral Corticosteroid-Dependent Asthma Patients., PMID:40243423
Effectiveness of Dupilumab and Omalizumab in Bullous Pemphigoid: A Nationwide Retrospective Cohort Study., PMID:40237419
Treatment of allergic bronchopulmonary aspergillosis with biologics., PMID:40226607
Utility of the Basophil Reactivity Test in the Clinical Management of People with Severe Uncontrolled Asthma., PMID:40224171
Sesame as a Food Allergen: Overview of Clinical Manifestations, Pathogenesis, Diagnosis and Treatment., PMID:40215130
Therapeutic Efficacy of YH35324 on FcεRIα-Mediated Mast Cell/Basophil Activation., PMID:40204504
Dual biological treatments in immune-mediated disorders: a single center experience., PMID:40200173
Managing Chronic Urticaria in Children: An Update., PMID:40192928
Vaccine-Induced Anti-IgE Antibodies Neutralize Free IgE but Fail to Bind and Activate Mast Cell-Displayed IgE., PMID:40192411
Emerging therapeutics in the management of food allergy., PMID:40189990
Drug survival of omalizumab in atopic asthma: Impact of clinical and genetic variables., PMID:40189906
Long-term, real-world effectiveness of biologics for severe uncontrolled asthma: The PROSPECT study., PMID:40186961
Combined use of Omalizumab and dupilumab: safety and efficacy data from a large academic center., PMID:40186636
Efficacy and Safety of Ligelizumab in Chronic Spontaneous Urticaria: A Systematic Review and Meta-analysis of Randomized Controlled Trials., PMID:40186041
[Omalizumab for the treatment of food allergies in adults]., PMID:40176616
[Indolent systemic mastocytosis : an overview in 2025]., PMID:40176613
Inequalities in Access to Specialist Allergy Services in the United Kingdom: A Report From the BSACI Registry for Immunotherapy (BRIT)., PMID:40169378
Evaluation of the efficacy of Omalizumab as re-treatment in patients with severe asthma., PMID:40169119
New insights into the mechanisms of childhood food allergies., PMID:40162591
Head-To-Head Comparison of Biologic Efficacy in Asthma: What Have We Learned?, PMID:40156481
Cost-Effectiveness Analysis of Omalizumab Combined With Standard of Care in Treating Moderate to Severe Asthma Children in China., PMID:40152080
Omalizumab and Oral Immunotherapy in IgE-Mediated Food Allergy in Children: A Systematic Review and a Meta-Analysis., PMID:40143213
Clinical efficacy and mechanisms of biologics for chronic rhinosinusitis with nasal polyps., PMID:40132672
Optimized Protein-Excipient Interactions in the Martini 3 Force Field., PMID:40129029