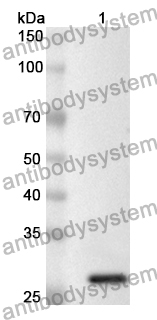
Catalog No.
PAD84001
Host species
Rabbit
Isotype
IgG
Clonality
Polyclonal
Immunogen
Ustekinumab
Tested applications
ELISA: 1:4000-1:8000
Target
Rabbit polyclonal to Stelara.
Specificity
The product is specific for Ustekinumab. This antibody serves as an excellent positive control for Ustekinumab immunogenicity (ADA) assays.
Concentration
0.5 mg/ml
Purification
Purified by antigen affinity column.
Accession
CAS: 815610-63-0
Applications
ELISA
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4, 50% Glycerol, 0.05% Proclin 300.
Stability and Storage
The product can be stored for 2 - 3 weeks at 2 to 8°C or for up to 12 months at -20°C. Avoid repeated freezing and thawing cycles.
Background
Ustekinumab with trade name Stelara, is an FDA-approved drug to Crohn's disease, Ulcerative Colitis, Plaque Psoriasis and Psoriatic Arthritis, targeting both interleukins (IL)-12 and IL-23. Ustekinumab is a fully human immunoglobulin G1 kappa mAb that binds to IL-12 and IL-23. Ustekinumab binds to the p40 subunit common to IL-12 and IL-23 and prevents their interaction with the IL-12 receptor β1 subunit of the IL-12 and IL-23 receptor complexes, thereby disrupting the proinflammatory pathway. Antibodysystem Anti-Ustekinumab Antibody, pAb, Rabbit is produced from sera of a rabbit immunized with Ustekinumab.
Treatment sequences, outcomes, healthcare utilization, and costs in patients with inflammatory bowel diseases requiring advanced treatment-real world comparative effectiveness from German claims data., PMID:40481399
Interleukin-23 Inhibitors for Inflammatory Bowel Disease: Pivotal Trials and Practical Considerations., PMID:40465057
Complete Resolution of Pityriasis Rubra Pilaris With Targeted Treatment: A Case Report., PMID:40400847
The 'totality of evidence' and 'extrapolation' of SB17, a ustekinumab biosimilar., PMID:40396611
Predicting ustekinumab treatment response in Crohn's disease using pre-treatment biopsy images., PMID:40366737
Anti-IL-12/23p40 antibodies for induction of remission in Crohn's disease., PMID:40357993
Cost per remission for mirikizumab versus ustekinumab for moderately to severely active ulcerative colitis treatment from the United States commercial payer perspective., PMID:40351121
Second-line strategies after anti-TNF failure in chronically active, moderate-to-severe ulcerative colitis: a retrospective, multicentre cohort study., PMID:40346848
Pharmacokinetic equivalence and comparative safety, tolerability, and immunogenicity of Biocon's ustekinumab (Bmab-1200) with EU-approved and US-licensed reference ustekinumab in healthy subjects: results from the Study to Test pharmacokinetic BioEquivalence of BiosimiLar ustekinumab to SteLARa (STELLAR-1)., PMID:40331766
Ustekinumab is effective in the treatment of linear psoriasis: a case report and literature review., PMID:40330485
The real-world effectiveness of ustekinumab in patients with ulcerative colitis in the United States., PMID:40327500
One-Year Efficacy of Guselkumab Versus Advanced Therapies for the Treatment of Moderately to Severely Active Crohn's Disease: A Network Meta-Analysis., PMID:40327280
Prevalence of opportunistic infections in Syrian inflammatory bowel disease patients on biologic therapy: a multi-center retrospective cross-sectional study., PMID:40320559
Cutaneous and systemic improvements in psoriasis patients after different biologic treatments in a real-world longitudinal prospective study., PMID:40319105
Efficacy and safety of Mirikizumab for ulcerative colitis: a systematic review and meta-analysis of randomized controlled trials., PMID:40301737
A multicenter study of the real-world effectiveness and safety of risankizumab in Crohn's disease., PMID:40289770
Impact of excess weight on clinical features of psoriasis and efficacy of biologic therapies in children with severe psoriasis: Analysis of data from the BiPe cohorts., PMID:40286464
New Therapeutic Challenges in Pediatric Gastroenterology: A Narrative Review., PMID:40281872
Relapsed refractory immune checkpoint inhibitor-induced colitis treated with tacrolimus and maintained with vedolizumab., PMID:40279047
The role of interleukin inhibitors in the treatment of hidradenitis suppurativa; a systematic review of clinical trials., PMID:40268126
Diffusion dimensionality modeling of subcutaneous/intramuscular absorption of antibodies and long-acting injectables., PMID:40263185
Triple biologic therapy for refractory Crohn's disease., PMID:40251965
Effects of biological agents on lipid profile and hemogram parameters in patients with psoriasis., PMID:40208364
Efficacy and safety of SYSA1902 versus reference ustekinumab in moderate-to-severe plaque psoriasis: A multicenter, randomized, phase III study., PMID:40208132
Real-World Effectiveness and Safety of Mirikizumab Induction Therapy in Patients With Ulcerative Colitis: A Multicentre Retrospective Observational Study., PMID:40205711
Successful Treatment of Ustekinumab-Associated Lichenoid Drug Eruption with Upadacitinib: A Case Report., PMID:40196725
Optimizing Maternal and Fetal Antibody Exposure and Dosing Regimens During Pregnancy Using a Physiologically Based Pharmacokinetic Model., PMID:40181734
Inflammatory bowel disease in a young female patient with a novel de novo TRAF3 frameshift variant responsive to ustekinumab: a case report., PMID:40181234
Summary of Research: Comparable Efficacy and Safety of Brodalumab in Obese and Nonobese Patients with Psoriasis: Analysis of Two Randomized Controlled Trials., PMID:40156694
Real-world effectiveness of ustekinumab versus anti-TNF or vedolizumab in ulcerative colitis: induction and 12-month maintenance results from the prospective, observational RUN-UC study., PMID:40152278
Comparative real-world effectiveness of ustekinumab versus anti-TNF in Crohn's disease: 12-month maintenance phase results from the prospective, observational RUN-CD study using propensity score adjustment., PMID:40132057
IL23p19 therapies for moderately-to-severely active ulcerative colitis., PMID:40082083
Allergy to ustekinumab: Validating skin tests for diagnostic and therapeutic decision-making., PMID:40051429
A Randomised, Double-Blind Trial to Compare the Efficacy, Safety, and Immunogenicity of the Biosimilar Ustekinumab FYB202 with Reference Ustekinumab in Patients with Moderate-to-Severe Plaque Psoriasis., PMID:40048101
Emerging treatments for dermatologic diseases in infants, children, and adolescents: a systematic review of clinical trials on biologics and small molecule inhibitors., PMID:40042725
Therapeutic Value of Exclusive and Partial Enteral Nutrition in Adult Patients with Moderate to Severe Crohn's Disease with Ustekinumab for Induction of Remission., PMID:40033533
Drug Survival of Biological Therapies in Smokers and Non-Smokers With Psoriasis: A Retrospective Cohort Study Using Data From the Australasian Psoriasis Registry., PMID:40028779
Ustekinumab Drug Clearance Is Better Associated with Disease Control than Serum Trough Concentrations in a Prospective Cohort of Inflammatory Bowel Disease., PMID:40006554
Effectiveness of biologics for endoscopic healing in patients with isolated proximal small bowel Crohn's disease., PMID:39991678
The safety assessment of ustekinumab on psoriasis and psoriatic arthritis: A real-world analysis based on the FDA adverse event reporting system database., PMID:39987634
Synergistic role of gut-microbial L-ornithine in enhancing ustekinumab efficacy for Crohn's disease., PMID:39978335
Ustekinumab for ulcerative colitis: a nationwide real-life observational cohort study., PMID:39970041
First-line anti-TNF agents, ustekinumab and vedolizumab perform similarly in Crohn' disease, but not in ulcerative colitis., PMID:39970039
Unlocking hope: The future of ustekinumab biosimilars in Crohn's disease treatment., PMID:39967304
Tumor necrosis factor (TNF) inhibitors for psoriatic arthritis., PMID:39945386
Body weight-range based initial dosing of ustekinumab in Crohn's disease: Is it an ideal approach?, PMID:39939201
Efficacy of Dose Escalation of Ustekinumab in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis., PMID:39900017
Ustekinumab is safe and effective in pediatric patients with Crohn's disease., PMID:39888083
First-line biologics as a treatment for ulcerative colitis: a multicenter randomized control study., PMID:39883201