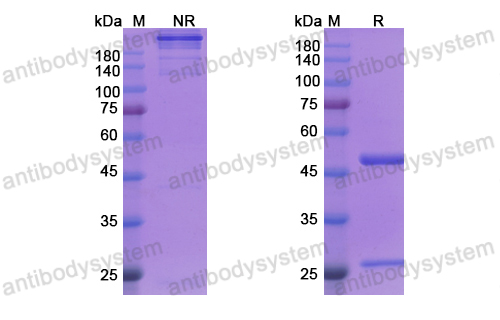
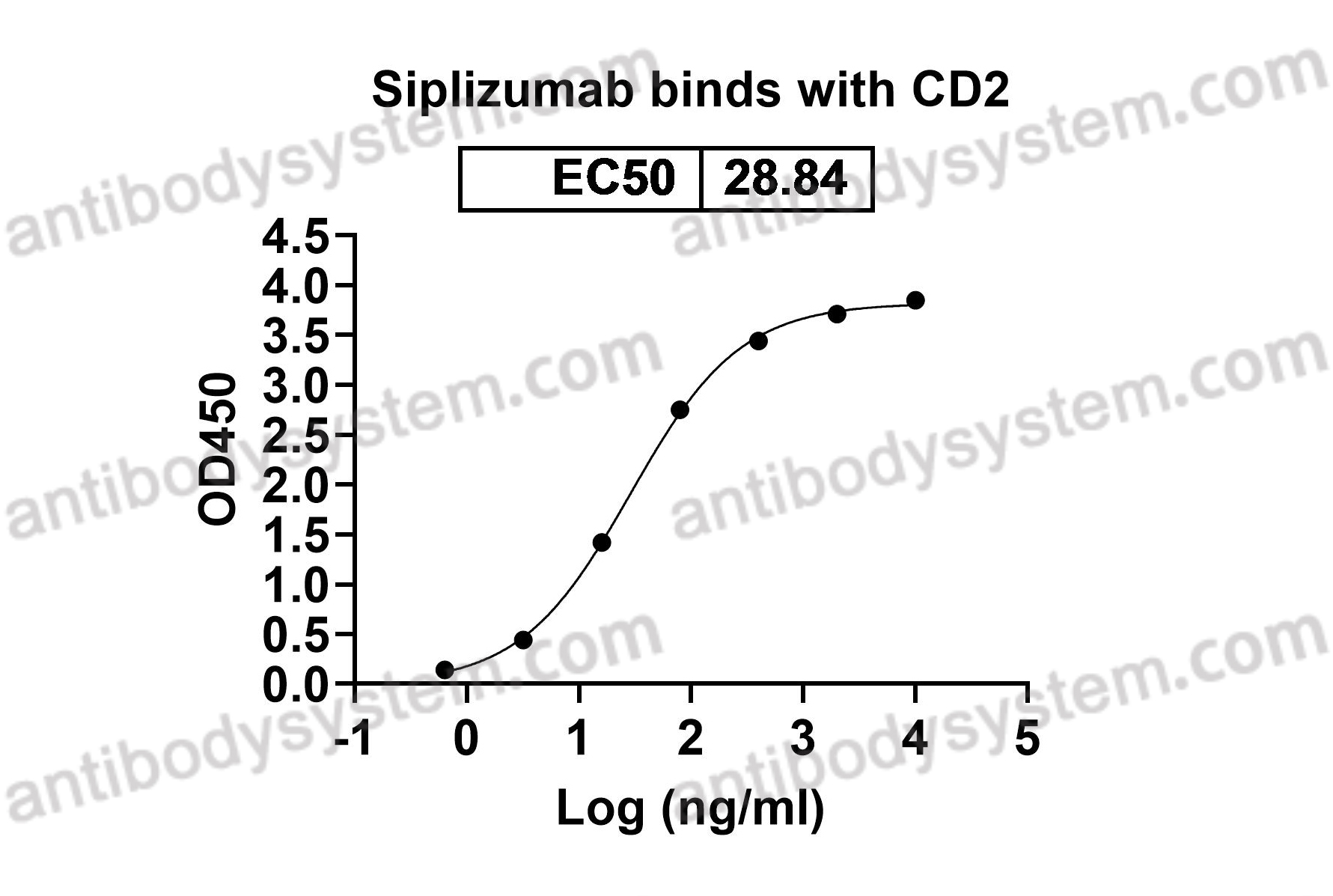
Catalog No.
DHC20801
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
Erythrocyte receptor, SRBC, T-cell surface antigen CD2, Rosette receptor, CD2, LFA-3 receptor, T-cell surface antigen T11/Leu-5, LFA-2
Concentration
2.93 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P06729
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
MEDI-507, CAS: 288392-69-8
Clone ID
Siplizumab
CD2 Immunobiology, PMID: 32582179
EBV-related lymphoproliferative disease complicating therapy with the anti-CD2 monoclonal antibody, siplizumab, in patients with T-cell malignancies, PMID: 19293260
Effective therapy for a murine model of adult T-cell leukemia with the humanized anti-CD2 monoclonal antibody, MEDI-507, PMID: 12649132
Gateways to clinical trials, PMID: 12224444
Gateways to clinical trials, PMID: 12949633
Humanized anti-CD2 monoclonal antibody treatment of plaque psoriasis: efficacy and pharmacodynamic results of two randomized, double-blind, placebo-controlled studies of intravenous and subcutaneous siplizumab, PMID: 19471949
Monoclonal antibodies in the treatment of non-Hodgkin's lymphoma, PMID: 17335294
Novel and emerging drugs for rarer chronic lymphoid leukaemias, PMID: 22483155
Pharmacokinetic and pharmacodynamic study of a clinically effective anti-CD2 monoclonal antibody, PMID: 31630416
Phase I dose escalation study of the anti-CD2 monoclonal antibody, siplizumab, with DA-EPOCH-R in aggressive peripheral T-cell lymphomas, PMID: 29032710
Phase-1 study of siplizumab in the treatment of pediatric patients with at least grade II newly diagnosed acute graft-versus-host disease, PMID: 19671093
Regulatory T-cell recovery in recipients of haploidentical nonmyeloablative hematopoietic cell transplantation with a humanized anti-CD2 mAb, MEDI-507, with or without fludarabine, PMID: 17588483
Safety profile and clinical outcomes in a phase I, placebo-controlled study of siplizumab in acute graft-versus-host disease, PMID: 19623014
Safety profile of intravenous and subcutaneous siplizumab, an anti-CD2 monoclonal antibody, for the treatment of plaque psoriasis: results of two randomized, double-blind, placebo-controlled studies, PMID: 20618506
Safety profile, pharmacokinetics, and pharmacodynamics of siplizumab, a humanized anti-CD2 monoclonal antibody, in renal allograft recipients, PMID: 19917362
Siplizumab Induces NK Cell Fratricide Through Antibody-Dependent Cell-Mediated Cytotoxicity, PMID: 33643309
Siplizumab selectively depletes effector memory T cells and promotes a relative expansion of alloreactive regulatory T cells in vitro, PMID: 31319439
Siplizumab, an Anti-CD2 Monoclonal Antibody, Induces a Unique Set of Immune Modulatory Effects Compared to Alemtuzumab and Rabbit Anti-Thymocyte Globulin In Vitro, PMID: 33262770
The anti-CD2 monoclonal antibody BTI-322 generates unresponsiveness by activation-associated T cell depletion, PMID: 15544625
Siplizumab combination therapy with belatacept or abatacept broadly inhibits human T cell alloreactivity in vitro., PMID:37270108
Distinct association between chronic Epstein-Barr virus infection and T cell compartments from pediatric heart, kidney, and liver transplant recipients., PMID:37187296
Immunotherapy in indolent Non-Hodgkin's Lymphoma., PMID:35663281
Siplizumab Induces NK Cell Fratricide Through Antibody-Dependent Cell-Mediated Cytotoxicity., PMID:33643309
Siplizumab, an Anti-CD2 Monoclonal Antibody, Induces a Unique Set of Immune Modulatory Effects Compared to Alemtuzumab and Rabbit Anti-Thymocyte Globulin In Vitro., PMID:33262770
CD2 Immunobiology., PMID:32582179
Pharmacokinetic and pharmacodynamic study of a clinically effective anti-CD2 monoclonal antibody., PMID:31630416
Siplizumab selectively depletes effector memory T cells and promotes a relative expansion of alloreactive regulatory T cells in vitro., PMID:31319439
Phase I dose escalation study of the anti-CD2 monoclonal antibody, siplizumab, with DA-EPOCH-R in aggressive peripheral T-cell lymphomas., PMID:29032710
Novel and emerging drugs for rarer chronic lymphoid leukaemias., PMID:22483155
Safety profile of intravenous and subcutaneous siplizumab, an anti-CD2 monoclonal antibody, for the treatment of plaque psoriasis: results of two randomized, double-blind, placebo-controlled studies., PMID:20618506
Safety profile, pharmacokinetics, and pharmacodynamics of siplizumab, a humanized anti-CD2 monoclonal antibody, in renal allograft recipients., PMID:19917362
Phase-1 study of siplizumab in the treatment of pediatric patients with at least grade II newly diagnosed acute graft-versus-host disease., PMID:19671093
Safety profile and clinical outcomes in a phase I, placebo-controlled study of siplizumab in acute graft-versus-host disease., PMID:19623014
Humanized anti-CD2 monoclonal antibody treatment of plaque psoriasis: efficacy and pharmacodynamic results of two randomized, double-blind, placebo-controlled studies of intravenous and subcutaneous siplizumab., PMID:19471949
EBV-related lymphoproliferative disease complicating therapy with the anti-CD2 monoclonal antibody, siplizumab, in patients with T-cell malignancies., PMID:19293260
Regulatory T-cell recovery in recipients of haploidentical nonmyeloablative hematopoietic cell transplantation with a humanized anti-CD2 mAb, MEDI-507, with or without fludarabine., PMID:17588483
Monoclonal antibodies in the treatment of non-Hodgkin's lymphoma., PMID:17335294
The anti-CD2 monoclonal antibody BTI-322 generates unresponsiveness by activation-associated T cell depletion., PMID:15544625
Gateways to clinical trials., PMID:12949633
Effective therapy for a murine model of adult T-cell leukemia with the humanized anti-CD2 monoclonal antibody, MEDI-507., PMID:12649132
Gateways to clinical trials., PMID:12224444