Catalog No.
DHJ36101
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG2-lambda
Clonality
Monoclonal
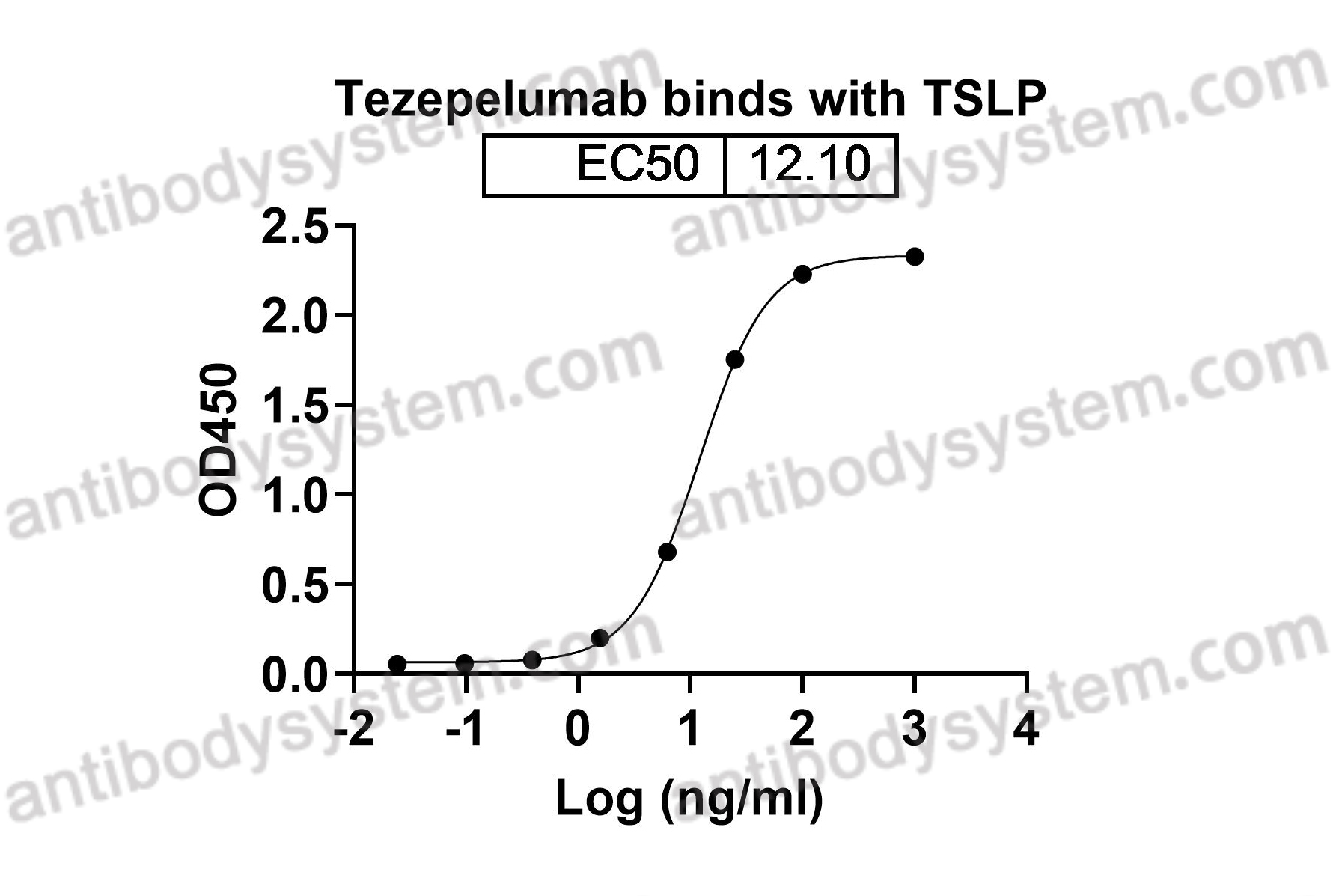
Target
TSLP, Thymic stromal lymphopoietin
Concentration
6.59 mg/ml
Endotoxin level
Please contact with the lab for this information.
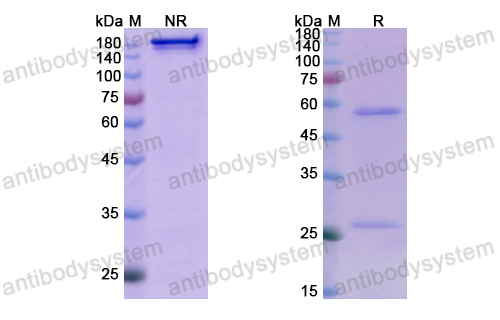
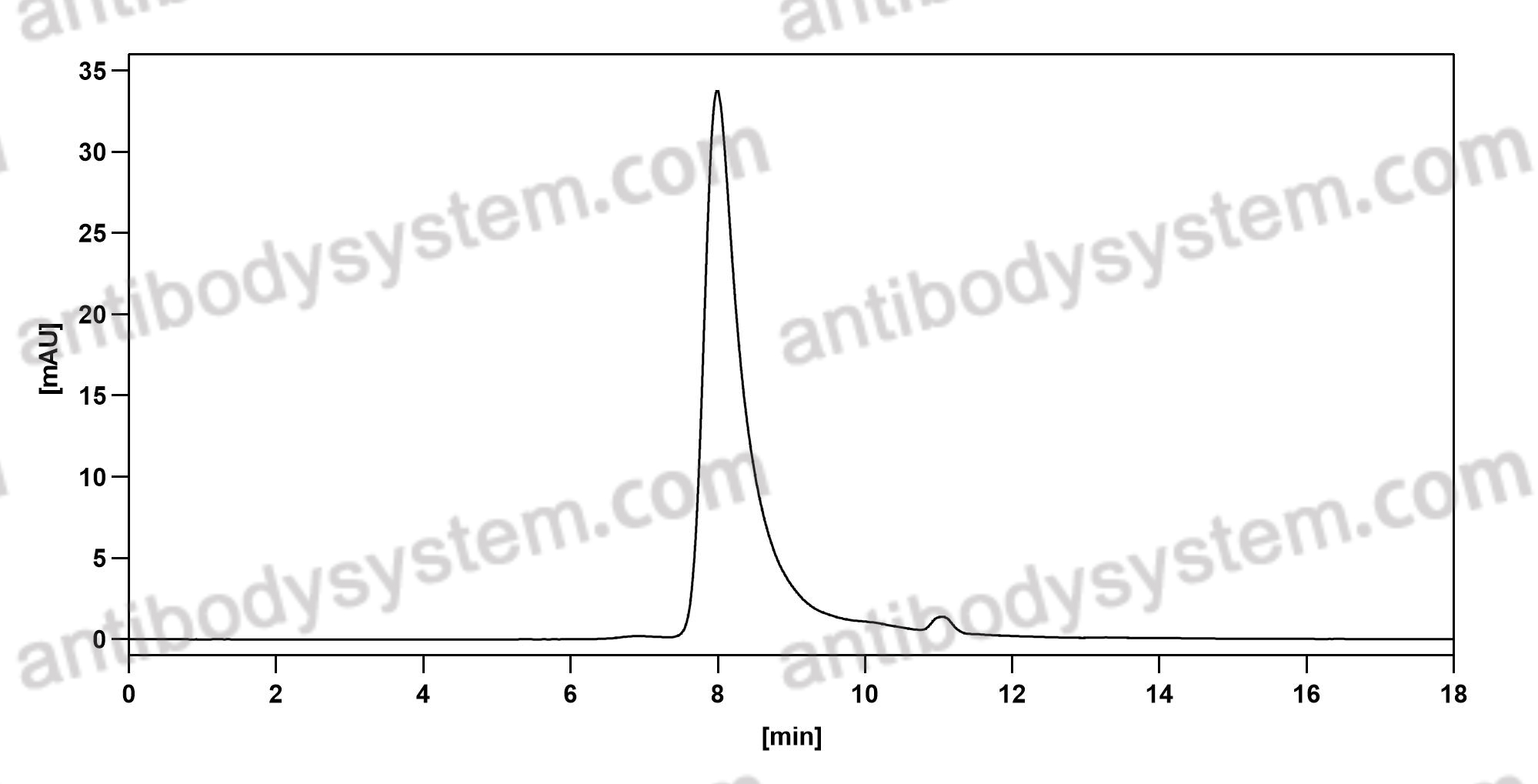
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q969D9
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
100 mM Pro-Ac, 20 mM Arg, pH 5.0
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
AMG 157, AMG-157, MEDI-9929, MEDI9929, anti-TSLP-Receptor (TSLP-R, Crl2), CAS: 1572943-04-4
Clone ID
Tezepelumab
[Alarmins and anti-alarmin biologics in asthma], PMID: 30246661
A Phase 1, Randomized, Placebo-Controlled Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Immunogenicity of Subcutaneous Tezepelumab in Healthy Japanese Men, PMID: 31960624
Antibodies to watch in 2021, PMID: 33459118
Anti-TSLP antibodies: Targeting a master regulator of type 2 immune responses, PMID: 31974038
Biological therapies for atopic dermatitis: An update, PMID: 30679974
Biologics for the Treatments of Allergic Conditions: Severe Asthma, PMID: 33012319
CASCADE: a phase 2, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the effect of tezepelumab on airway inflammation in patients with uncontrolled asthma, PMID: 33050900
DESTINATION: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the long-term safety and tolerability of tezepelumab in adults and adolescents with severe, uncontrolled asthma, PMID: 33087119
Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial, PMID: 34256031
Efficacy of biologics in atopic dermatitis, PMID: 32003247
Efficacy of Tezepelumab in Patients with Severe, Uncontrolled Asthma and Perennial Allergy, PMID: 34358701
Efficacy of Tezepelumab in Patients with Severe, Uncontrolled Asthma with and without Nasal Polyposis: A Post Hoc Analysis of the Phase 2b PATHWAY Study, PMID: 33568920
Emerging Treatment Options in Atopic Dermatitis: Systemic Therapies, PMID: 29320765
Functionality and Performance of an Accessorized Pre-Filled Syringe and an Autoinjector for At-Home Administration of Tezepelumab in Patients with Severe, Uncontrolled Asthma, PMID: 33907423
Immunobiologic treatments for severe asthma, atopic dermatitis, and chronic urticaria, PMID: 31690400
Molecular Targets for Biological Therapies of Severe Asthma: Focus on Benralizumab and Tezepelumab, PMID: 34440488
Monoclonal antibodies in type 2 asthma: a systematic review and network meta-analysis, PMID: 31395084
NAVIGATOR: a phase 3 multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the efficacy and safety of tezepelumab in adults and adolescents with severe, uncontrolled asthma, PMID: 33050934
New Anti-Eosinophil Drugs for Asthma and COPD: Targeting the Trait!, PMID: 28583618
New perspectives of childhood asthma treatment with biologics, PMID: 30444939
New Targeted Therapies for Uncontrolled Asthma, PMID: 31076057
Novel Therapeutic Approaches and Targets for the Treatment of Atopic Dermatitis, PMID: 32525769
Pharmacokinetic and Pharmacodynamic Modeling of Tezepelumab to Guide Phase 3 Dose Selection for Patients With Severe Asthma, PMID: 33368307
Pharmacokinetics, Safety, and Tolerability of Tezepelumab (AMG 157) in Healthy and Atopic Dermatitis Adult Subjects, PMID: 30779339
Review and analysis of biologic therapies currently in phase II and phase III clinical trials for atopic dermatitis, PMID: 32507066
Revisiting Therapies for Atopic Dermatitis that Failed Clinical Trials, PMID: 32172523
Severe asthma: what is new in the new millennium, PMID: 32004177
SOURCE: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel group trial to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma, PMID: 33050928
Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma, PMID: 28368013
Targeted Molecular Therapies in Allergy and Rhinology, PMID: 33138725
T-cell inhibitors for atopic dermatitis, PMID: 29248519
Tezepelumab as an Emerging Therapeutic Option for the Treatment of Severe Asthma: Evidence to Date, PMID: 33536746
Tezepelumab improves patient-reported outcomes in patients with severe, uncontrolled asthma in PATHWAY, PMID: 33169672
Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma, PMID: 33979488
Tezepelumab in Adults with Uncontrolled Asthma, PMID: 28877011
Tezepelumab in Adults with Uncontrolled Asthma, PMID: 31034183
Tezepelumab normalizes serum interleukin-5 and -13 levels in patients with severe, uncontrolled asthma, PMID: 34403803
Tezepelumab Pharmacokinetics, Safety, and Tolerability After Administration via Vial-and-syringe, Accessorized Prefilled Syringe, or Autoinjector: A Randomized Trial in Healthy Volunteers, PMID: 33380362
Tezepelumab prepares to enter the asthma antibody fray, PMID: 33441998
Tezepelumab Reduces Exacerbations Across All Seasons in Patients with Severe, Uncontrolled Asthma: A Post Hoc Analysis of the PATHWAY Phase 2b Study, PMID: 33469316
Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial, PMID: 30550828
Tezepelumab: a novel biological therapy for the treatment of severe uncontrolled asthma, PMID: 31549891
Tezepelumab: A Potential New Biological Therapy for Severe Refractory Asthma, PMID: 33922072
The effect of tezepelumab on airway hyperresponsiveness to mannitol in asthma (UPSTREAM), PMID: 34049943
The effect of tezepelumab on hospitalizations and emergency department visits in patients with severe asthma, PMID: 32474159
Thymic Stromal Lymphopoietin Isoforms, Inflammatory Disorders, and Cancer, PMID: 30057581
Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma, PMID: 32567399
TSLP Inhibitors for Asthma: Current Status and Future Prospects, PMID: 32078149
Unmet need in severe, uncontrolled asthma: can anti-TSLP therapy with tezepelumab provide a valuable new treatment option?, PMID: 33059715
What's New in Atopic Dermatitis, PMID: 30850043