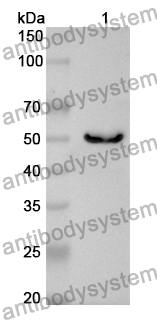
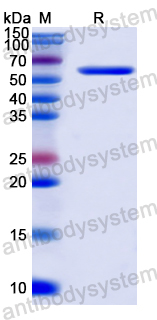
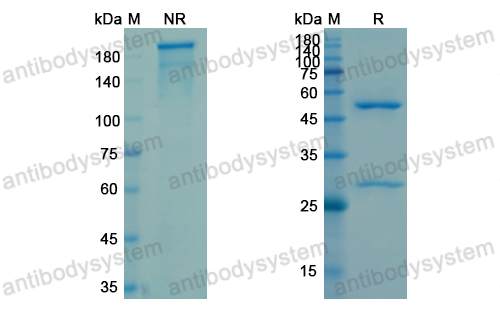
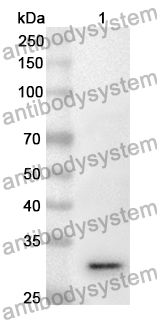
Anti-Tumor Activities of Anti-Siglec-15 Chimeric Heavy-Chain Antibodies., PMID:40507880
Detectable SARS-CoV-2 specific immune responses in recovered unvaccinated individuals 250 days post wild type infection., PMID:40498720
AeroVax: study protocol for a randomised, double-blind, placebo-controlled, phase 2 trial to evaluate safety and immunogenicity of a next-generation COVID-19 vaccine delivered by inhaled aerosol to humans., PMID:40491461
Safety and efficacy of systemic chemotherapy plus PD-1 inhibitor in combination with intravenous or intraperitoneal bevacizumab in gastric cancer with peritoneal metastasis., PMID:40481442
pH-Responsive Diblock Copolymer Vesicles via Polymerization-Induced Self-Assembly in Aqueous Media: Synthesis, Loading, and Potential Biological Applications., PMID:40460265
Allostery Links hACE2 Binding, Pan-variant Neutralization and Helical Extension in the SARS-CoV-2 Spike Protein., PMID:40447075
Long-Term Dynamics of SARS-CoV-2 Variant-Specific Neutralizing Antibodies Following mRNA Vaccination and Infection., PMID:40431687
Accelerating Biologics PBPK Modelling with Automated Model Building: A Tutorial., PMID:40430895
Physiologically Based Pharmacokinetic Modeling of Biologic Case Studies in Monkeys and Humans Reveals the Necessity of an Additional Clearance Term., PMID:40430853
Development of a self-assembling multimeric Bann-RBD fusion protein in Pichia pastoris as a potential COVID-19 vaccine candidate., PMID:40425664
A combined digital microfluidic test for assessing infection and immunity status for viral disease in saliva., PMID:40423685
mRNA COVID-19 vaccines induce superior IgA titers in cancer patients compared to viral vector vaccines: Implications for immunization strategies., PMID:40414552
[Short-term effects and safety outcomes of the combination of tislelizumab and neoadjuvant chemotherapy in the perioperative treatment of locally advanced gastric cancer]., PMID:40404373
Designing tunable carbon nanotube-based nanomechanical biosensor for SARS-CoV-2 detection: A stochastic finite element analysis., PMID:40403628
Longitudinal assessment of COVID-19 vaccine immunogenicity in people with HIV stratified by CD4+ T-cell count in the Netherlands: A two-year follow-up study., PMID:40388467
A novel chimpanzee adenovirus vector vaccine for protection against infectious bronchitis and Newcastle disease in chickens., PMID:40375108
Electromagnetic waves destabilize the SARS-CoV-2 Spike protein and reduce SARS-CoV-2 Virus-Like particle (SC2-VLP) infectivity., PMID:40374718
Сomprehensive approach to comparative characteristics of clinical and laboratory parameters of the study in children - in 6 months after Covid-19 treatment and 6 months after Covid-19 vaccination., PMID:40367469
A four-week study on the toxicity of repeated intramuscular administration of plant-based BA-CoV2-0301 vaccine against SARS-CoV-2 in Sprague-Dawley rats., PMID:40366666
Comprehensive analysis of human coronavirus antibody responses in ICU and non-ICU COVID-19 patients reveals IgG3 against SARS-CoV-2 spike protein as a key biomarker of disease severity., PMID:40359129
Detection of S1 spike protein in CD16+ monocytes up to 245 days in SARS-CoV-2-negative post-COVID-19 vaccine syndrome (PCVS) individuals., PMID:40358138
Evaluation of an E. coli-expressed spike protein-based in-house ELISA system for assessment of antibody responses after COVID-19 infection and vaccination., PMID:40352206
mRNA-delivered neutralizing antibodies confer protection against SARS-CoV-2 variant in the lower and upper respiratory tract., PMID:40342969
Using SARS-CoV-2 red cell kodecytes to assess vaccine-induced immune response to the conserved 1147-58 region of the spike protein in Indian blood donors: exploring the potential role of blood transfusion services in population surveillance., PMID:40335014
Identification of a nanobody able to catalyze the destruction of the spike-trimer of SARS-CoV-2., PMID:40317451
Revisiting resectability of biliary tract cancers, in the triplet drug therapy era with immune checkpoint inhibitors., PMID:40314879
A distinctive IGHV3-66 SARS-CoV-2 neutralizing antibody elicited by primary infection with an Omicron variant., PMID:40306272
SARS-CoV-2 S1 protein induces IgG-mediated platelet activation and is prevented by 1.8-cineole., PMID:40306177
Impact of Nirsevimab on RSV and Non-RSV Severe Respiratory Infections in Hospitalized Infants., PMID:40302169
Aptamer Paper-Based Fluorescent Sensor for Determination of SARS-CoV-2 Spike Protein., PMID:40292706
The recombinant immunodominant regions 179-344 and 550-670 from SARS-COV2 spike protein can efficiently react with patients' sera., PMID:40287104
Identification of B-Cell Epitopes Located on the Surface of the S1 Protein of Infectious Bronchitis Virus M41 Strains., PMID:40284907
Immune Imprinting, Non-Durable Hybrid Immunity, and Hybrid Immune Damping Following SARS-CoV-2 Primary Vaccination with BNT162b2 and Boosting with mRNA-1273., PMID:40266217
The Pseudotyped Replication-Deficient VSV with Spike from PEDV Induces Neutralizing Antibody Against PEDV., PMID:40266086
CoVimmune COVID-19 Immunity Calculator: Web Application Development and Validation Study., PMID:40262279
Enterococcus hirae-Associated Endocarditis Outbreak in Young Broiler Breeders of the Female Line., PMID:40249584
An Assessment of the Currently Available Molecular Assay for the Diagnosis of Anisakis Sensitization., PMID:40243680
Isolation and characterization of GI-19/L1148-like infectious bronchitis virus in China., PMID:40233840
Bivalent circular RNA vaccines against porcine epidemic diarrhea virus and transmissible gastroenteritis virus., PMID:40230842
Circulating Food Allergen-Specific Antibodies, Beyond IgG4, Are Elevated in Eosinophilic Esophagitis., PMID:40230181
Immunogenicity and protective efficacy of seven candidate vaccines boosted with recombinant proteins, whole-cell bacterins of three serotypes of Mannheimia haemolytica, and an emulsion-type adjuvant., PMID:40225758
A phase I/II trial evaluating the safety of increased-dose S- 1 with oxaliplatin and nivolumab in HER2-negative advanced gastric cancer., PMID:40221699
Robust immune responses to intranasal vaccine targeting Bordetella pertussis antigens to claudin-4 on mucosal microfold cells., PMID:40220802
Continuous, Label-Free Phenotyping of Single Cells Based on Antibody Interaction Profiling in Microfluidic Channels., PMID:40220345
[The highlights of nephrology in 2024]., PMID:40208671
[The highlights of kidney transplantation in 2024]., PMID:40208670
[PORK-CAT SYNDROME WITH ALLERGIC SYMPTOMS DUE TO THE INGESTION OF COW'S MILK]., PMID:40204486
Capture of fusion-intermediate conformations of SARS-CoV-2 spike requires receptor binding and cleavage at either the S1/S2 or S2' site., PMID:40198676
Intravital imaging of peritubular microcirculation impairment in cisplatin-induced acute kidney injury., PMID:40198123
A high-throughput, fully automated competition assay to evaluate SARS-CoV-2 neutralizing responses and epitope specificity in clinical samples., PMID:40185856